 Isomers are molecules that have the same molecular formula but different arrangements of the atoms. Structural isomers (see Fundamentals of organic chemistry 10.1) have different arrangements of the bonds in the molecule. Stereoisomers have the same bonds, but different arrangements of the atoms in three-dimensional space. This is a short section, but there is a lot of new vocabulary to get the head around.
Isomers are molecules that have the same molecular formula but different arrangements of the atoms. Structural isomers (see Fundamentals of organic chemistry 10.1) have different arrangements of the bonds in the molecule. Stereoisomers have the same bonds, but different arrangements of the atoms in three-dimensional space. This is a short section, but there is a lot of new vocabulary to get the head around.
Ensure you are confident using the terms below and learn the asterisked* definitions
Cis or Z (zusammen) isomers, Trans or E (entgegen) isomers, Enantiomers*, A chiral carbon (chiral centre)*, Optical activity, A racemic mixture (racemate)*
Which compound can show optical activity?
Optical activity is shown by compounds with mirror image isomers (enantiomers). In order to be optically active, compounds must contain a chiral carbon atom - that is a carbon atom with four different substituents attached.
The second carbon atom as written in butan-2-ol, CH3CHOHCH2CH3 has four different substituents attached (CH3, H, OH and C2H5) and so is chiral.
The other compounds do not have a chiral carbon.
Therefore CH3CHOHCH2CH3 is the correct answer.
Which compound can show optical activity?
Optical activity is shown by compounds with mirror image isomers (enantiomers). In order to be optically active, compounds must contain a chiral carbon atom - that is a carbon atom with four different substituents attached.
The second carbon atom as written in 2-chloro-3-methylbutane, CH3CClHCH(CH3)2 has four different substituents attached (CH3, Cl, H and CH(CH3)2) and so is chiral.
The other compounds do not have a chiral carbon.
Therefore 2-chloro-3-methylbutane is the correct answer.
Which compound shows cis-trans isomerism?
Cis-trans isomerism is shown by compounds when the two substituents attached to both of the carbon atoms of the C=C double bond are different.
In but-2-ene, CH3CHCHCH3 the first carbon of the double bond has CH3 and H attached, as does the second carbon of the double bond, so but-2-ene will show cis-trans isomerism.
The other compounds all have two substituents that are the same attached to one of the carbon atoms of the double bond.
Therefore CH3CHCHCH3 is the correct answer.
Which compound shows cis-trans isomerism?
Cis-trans isomerism is shown by compounds when the two substituents attached to both of the carbon atoms of the C=C double bond are different.
In pent-2-ene, CH3CHCHCH2CH3 the first carbon of the double bond has CH3 and H attached, and the second carbon of the double bond has H and CH2CH3 attached, so pent-2-ene will show cis-trans isomerism.
The other compounds all have two substituents that are the same attached to one of the carbon atoms of the double bond.
Therefore pent-2-ene is the correct answer.
How many chiral carbon atoms are present in this molecule?
The grey atoms represent carbon, blue represent nitrogen, red represent oxygen, white represent hydrogen.
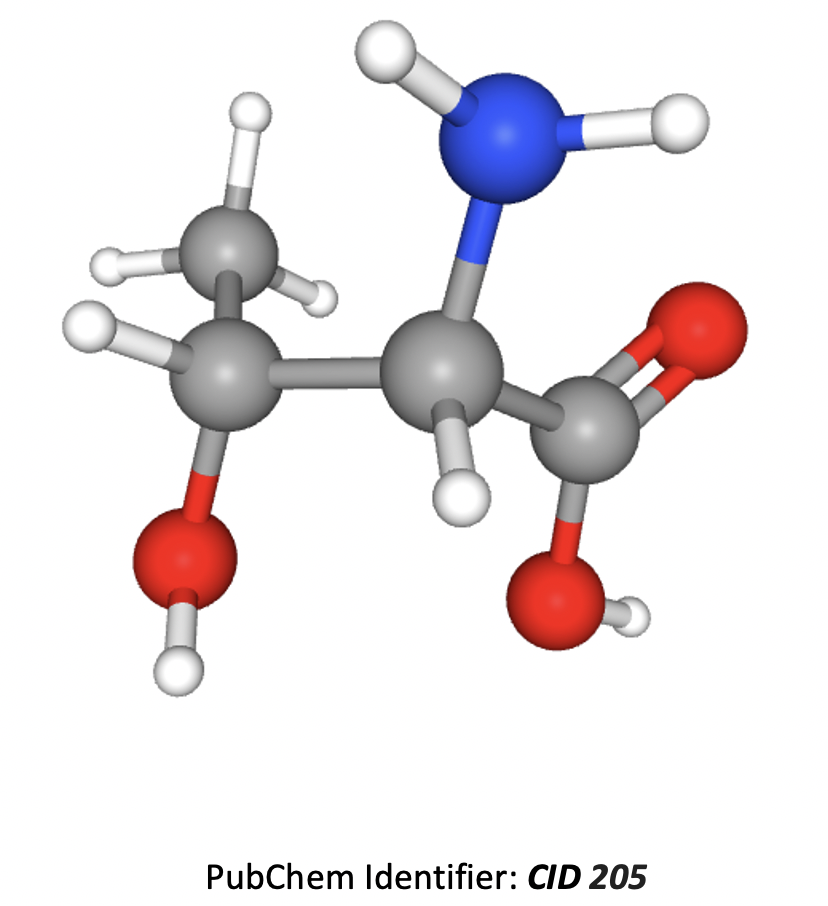
A chiral carbon atom, is a carbon atom with four different substituents attached.
The molecule is 2-amino-3-hydroxybutanoic acid. The second and third carbon atoms have four different substituents attached and so are chiral. The first carbon (CH3) and the last (CO2H) do not, so are not chiral (any carbon in a double bond cannot be chiral).
Therefore 2 is the correct answer.
Which of the following compounds show/s cis-trans isomerism?
1: 1,1-dichlorobut-1-ene
2: 1,2-dichlorobut-1-ene
3: 1,2-dichlorocyclobutane
Cis-trans isomerism is shown by compounds when the two substituents attached to both of the carbon atoms of the C=C double bond are different.
It can also be shown in cyclic compounds with two substituents attached to two different carbon atoms of the carbon ring.
1,1-dichlorobut-1-ene has two chlorine atoms attached to the first carbon atom of the double bond, so does not show cis-trans isomerism.
In 1,2-dichlorobut-1-ene the first carbon of the double bond has Cl and H attached, and the second carbon of the double bond has Cl and CH2CH3 attached, so 1,2-dichlorobut-1-ene will show cis-trans isomerism.
1,2-dichlorocyclobutane is a cyclic compound with two substituents attached to two different carbon atoms of the carbon ring so will show cis-trans isomerism. Trans-1,2-dichlorocyclobutane shown below:
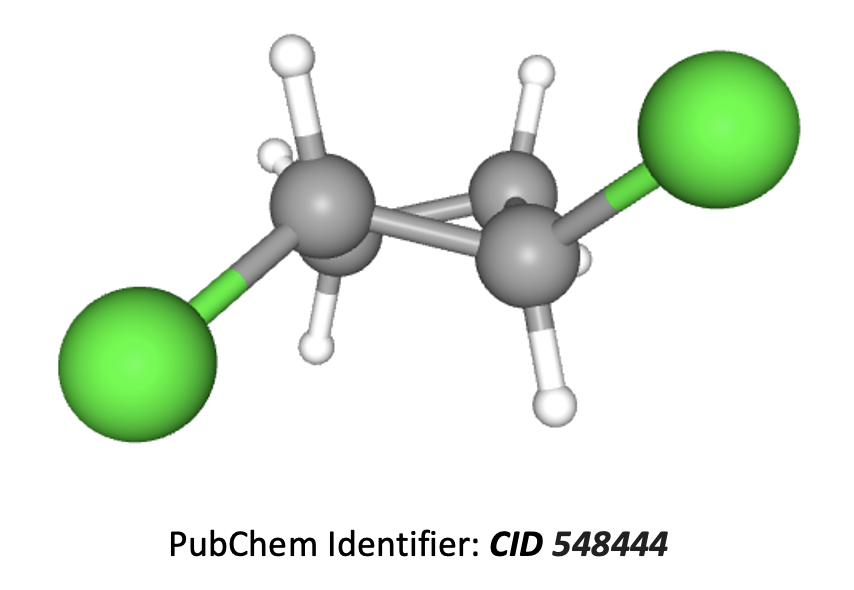
Therefore 2 and 3 only is the correct answer.
The compound shown below, 1,2-dichlorobut-2-ene, shows E/Z isomerism.
What are the priorities of the four substituents attached to the C=C double bond (A to D) as shown on the diagram?
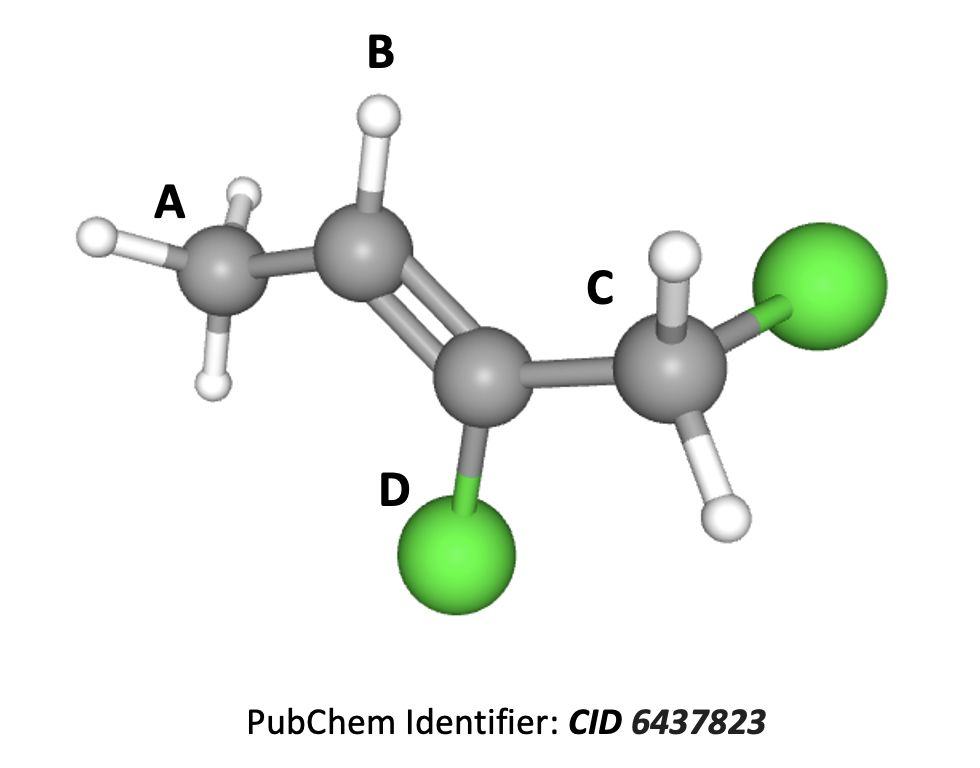
In E/Z isomerism each substituent is given a priority according to the atomic number (Z) of the atom attached to the carbon. Moving to the next atom in the event of a ‘draw’. (The highest substituents on each carbon atom are then compared to see if they are E (entgegen) or Z (zusammen) relative to one-another.)
A carbon Z=6, then hydrogen Z=1
B hydrogen Z=1
C carbon Z=6, then chlorine Z=17
D chlorine Z=17
So 1st D, 2nd C, 3rd A, 4th B is therefore the correct answer.
The compound shown below, 2-chlorobutenedioic acid, shows E/Z isomerism.
Does this diagram show the E or Z isomer, and which two of the four substituents are the highest priority on each carbon and therefore responsible for deciding whether this isomer is E or Z?
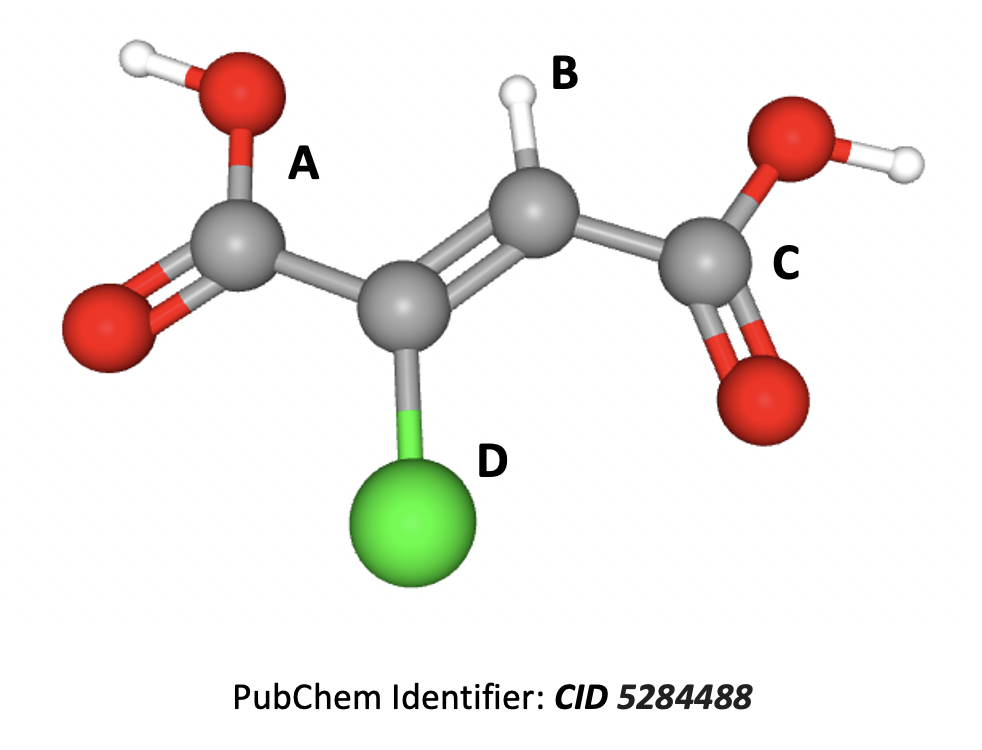
In E/Z isomerism each substituent is given a priority according to the atomic number (Z) of the atom attached to the carbon. Moving to the next atom in the event of a ‘draw’. The highest substituents on each carbon atom are then compared to see if they are E (entgegen) or Z (zusammen) relative to one-another.
A carbon Z=6, then oxygen Z=8
B hydrogen Z=1
C carbon Z=6, then oxygen Z=8
D chlorine Z=17
So D is the highest priority on the first carbon of the double bond, and C is the highest on the second carbon of the double bond.
D and C are 'together' on the same side of the carbon chain in the zusammen (Z) position.
Therefore Z isomer due to C and D is the correct answer.
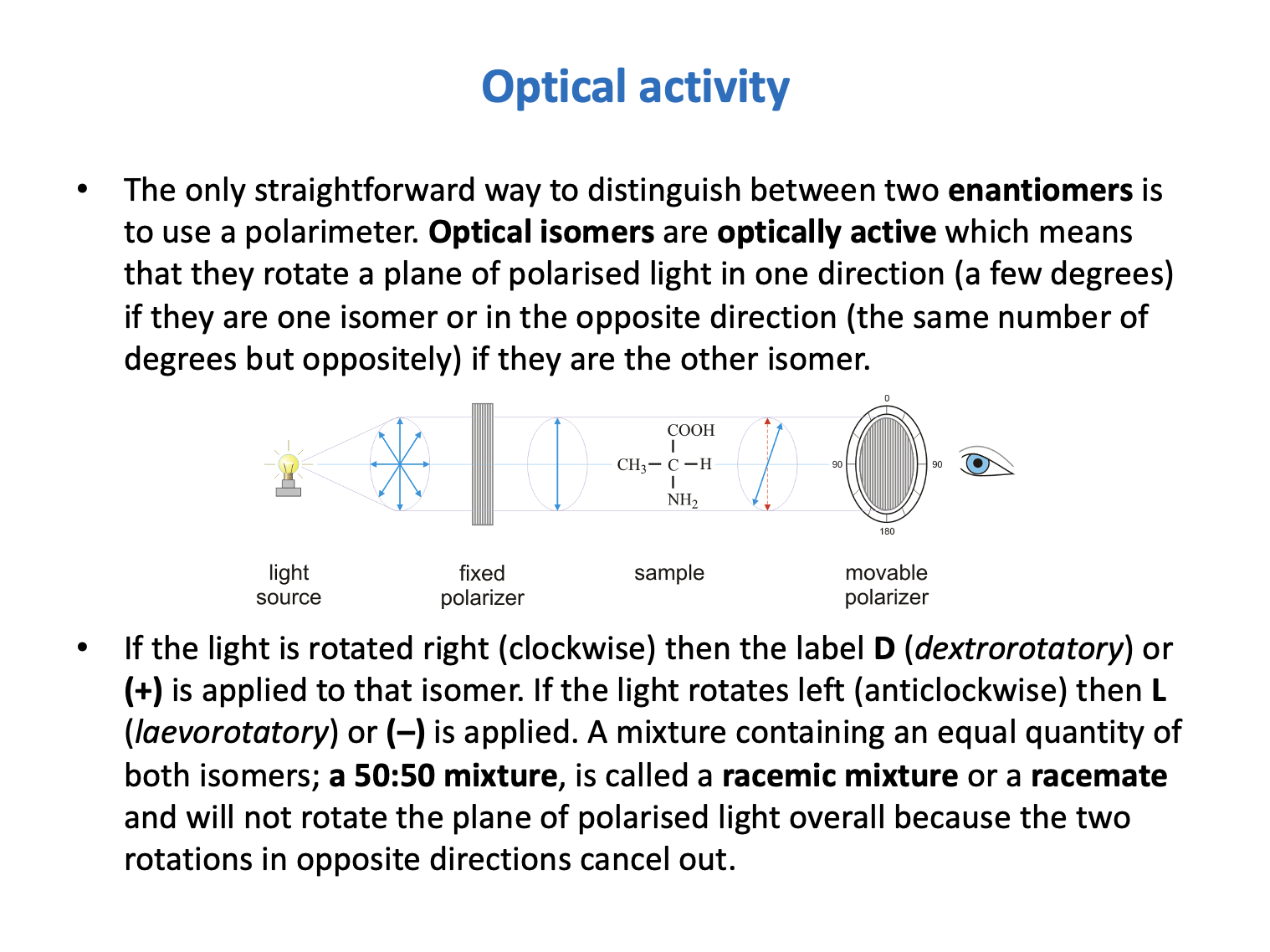
Butan-2-ol shows optical activity. Which of the following statements are correct?
1: A racemic mixture of butan-2-ol does not rotate plane polarised light.
2: A pure sample of (+)butan-2-ol rotates plane polarised light clockwise (to the right).
3: (+)butan-2-ol and (−)butan-2-ol have the same chemical properties.
Optical activity is shown by compounds with mirror image isomers (enantiomers). In order to be optically active, compounds must contain a chiral carbon atom - that is a carbon atom with four different substituents attached.
Enantiomers will rotate plane polarised light in different directions (by an equal but opposite amount).
(+) or D isomers rotate the light to the right (clockwise). (−) or L isomers rotate the light to the left (anticlockwise).
A 50:50 mixture, called a racemic mixture, will not rotate light as the rotations cancel out.
Enantiomers have the same chemical and physical properties (apart from the rotation of plane polarised light), unless reacting with a chiral binding site in a living organism.
Therefore 1, 2 and 3 is the correct answer.
Paper 1
Core (SL&HL): Organic chemistry core (SL and HL) paper 1 questions
AHL (HL only): Organic chemistry AHL (HL only) paper 1 questions
Paper 2
Core (SL&HL): Organic chemistry core (SL & HL) paper 2 questions
AHL (HL only): Organic chemistry AHL (HL only) paper 2 questions
How much of Stereoisomerism have you understood?













 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn