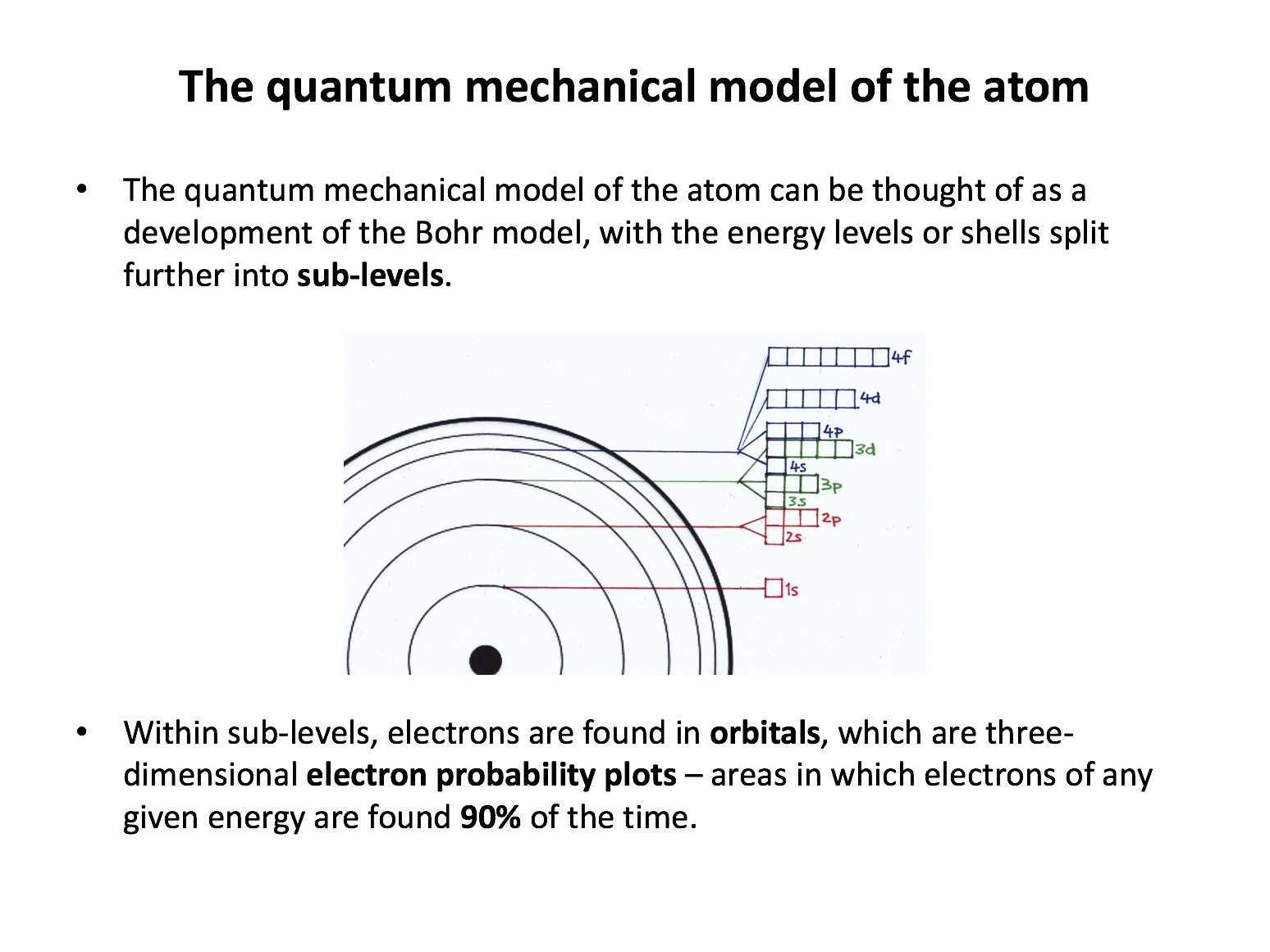
 The electron configuration, that is the distribution or arrangement of electrons in the atom, can be described by the Bohr model of the atom and the quantum mechanical model of the atom. The Bohr model is simpler, with electrons found in shells around the nucleus. The quantum mechanical model is more complex and takes into account a broader range of evidence that we have about the positions of electrons in atoms. You need to know both models of the atom. Although questions focused on electron configuration will always be asking about the quantum mechanical model (and that is what is tested in questions here), you also need to be confident in understanding the Bohr model, which is used in describing chemical bonding and structure, later in the course.
The electron configuration, that is the distribution or arrangement of electrons in the atom, can be described by the Bohr model of the atom and the quantum mechanical model of the atom. The Bohr model is simpler, with electrons found in shells around the nucleus. The quantum mechanical model is more complex and takes into account a broader range of evidence that we have about the positions of electrons in atoms. You need to know both models of the atom. Although questions focused on electron configuration will always be asking about the quantum mechanical model (and that is what is tested in questions here), you also need to be confident in understanding the Bohr model, which is used in describing chemical bonding and structure, later in the course.
This topic is probably the most challenging in the whole course in terms of the abstract concepts covered, so do not be worried if understanding it all is difficult at the start. Breaking it down by learning the key information presented in the revision cards and repeatedly test yourself using the practice questions will help you get there in the end.
Ensure you are confident using the terms below and learn the asterisked* definitions
emission spectrum, absorption spectrum, continuous spectrum*, line spectrum*, energy level, sub-level, orbital, Aufbau principle, Hund's rule, Pauli exclusion principle
The electronic energy levels in an atom of hydrogen are:
The electronic energy levels are further apart at lower energy - nearer the nucleus. They get closer together in energy at higher energy - further away from the nucleus.
The electron transition between which two atomic energy levels emits the most energy?
The question asks about emission - energy given out - so the transition must be from a higher to lower energy level. And as the energy gaps between the levels gets smaller at higher energy, third to first will be greater than sixth to fourth, so third to first is the correct answer.
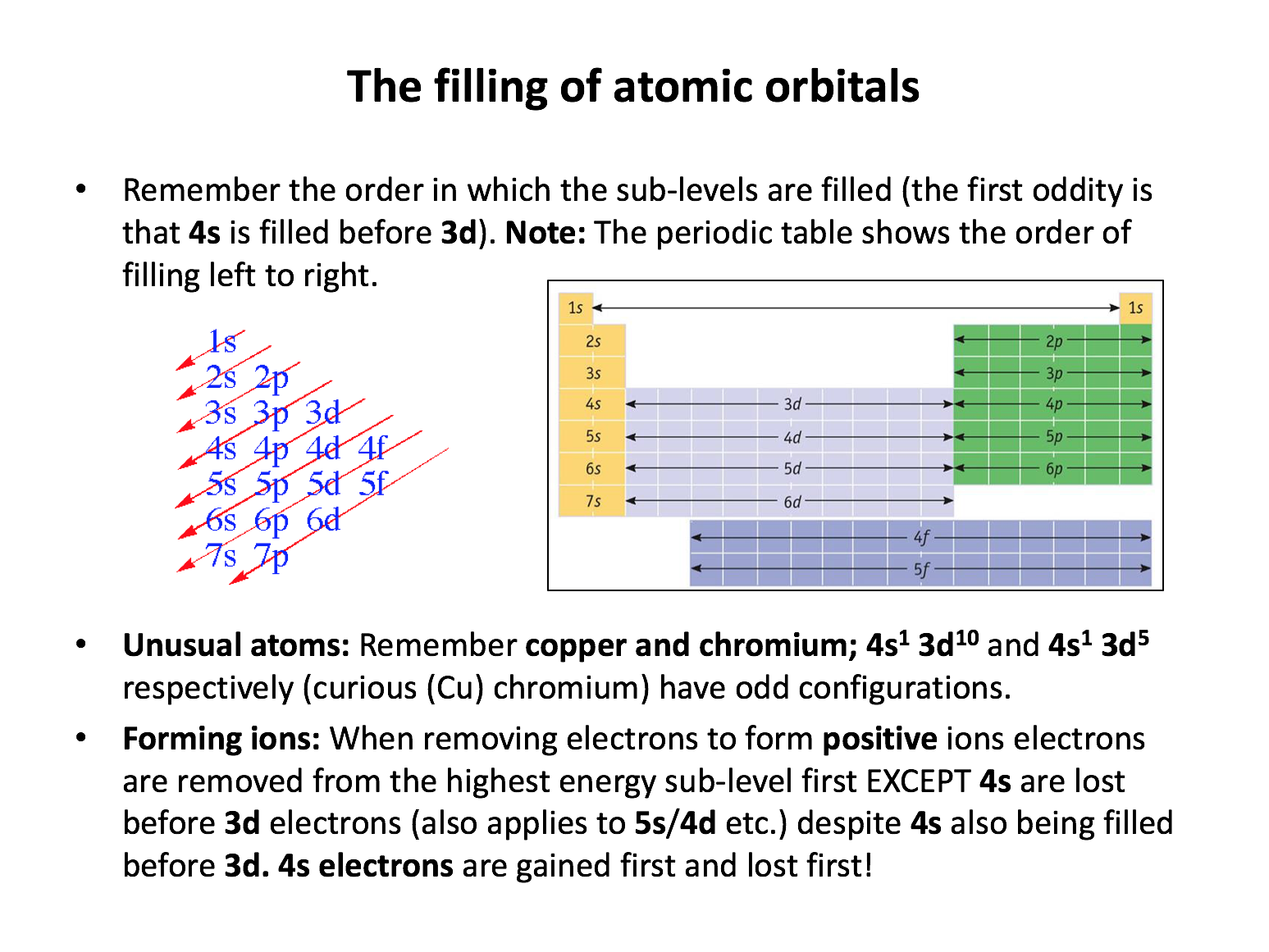
What is the correct order of filling of electronic energy sub-levels in an atom (up to atomic number 36)?
The order of filling is 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p. This must be learned. The periodic table can help since it is arranged in accordance with the filling of the electronic energy sub-levels in atoms.
What is the correct electronic configuration for an atom of sulfur?
The sub-levels of an atom are filled in the following order: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s. Remember that 's' sub-levels hold a maximum of 2 electrons, 'p' hold 6, and 'd' hold 10. A sulfur atom has sixteen electrons in total (atomic number is 16) so the electronic configuration for the sulfur atom is 1s2 2s2 2p6 3s2 3p4 .
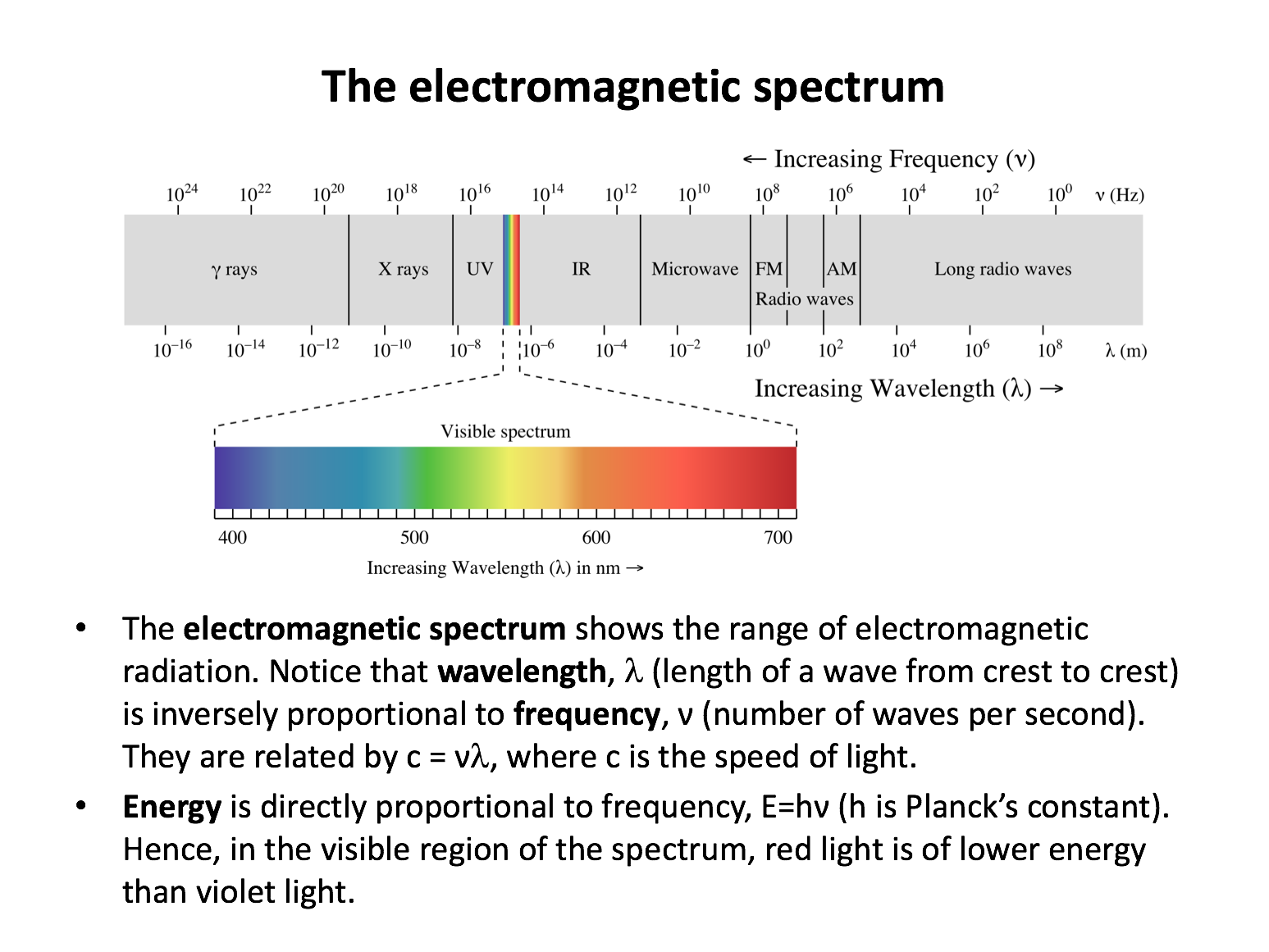
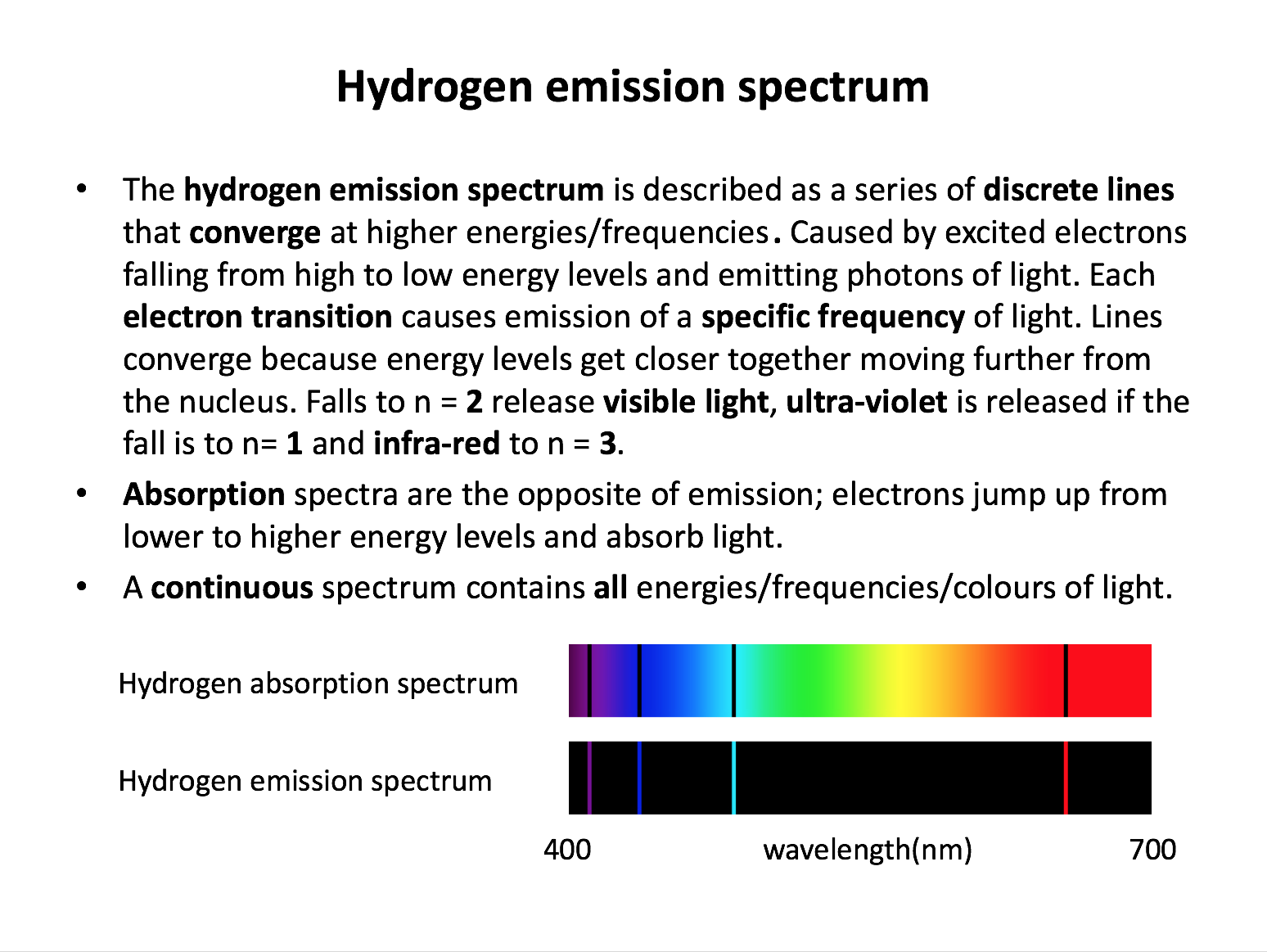
Electron transitions in the hydrogen emission spectrum to levels n=1, n=2 and n=3 generate lines in which parts of the electromagnetic spectrum respectively?
Electron transitions in the hydrogen emission spectrum from higher energy levels to n=1 result in emissions in the ultra-violet region (highest energy since these transitions have the largest gaps between energy levels). Transitions to n=2 are found in the visible region. Transitions to n=3 are found in the infra-red region (lowest energy since these transitions have the smallest gaps between energy levels).
Which description best describes the shape of a p orbital?
Orbitals are plots of electron position (90% probability) around the nucleus. They are three dimensional (not planar). An s orbital is a sphere; a p orbital is a three dimensional figure-of-eight. So the answer here that best decribes a p orbital is 'two spheres side by side (touching)'.
An atomic emission spectrum is produced when:
Electron transitions (movements) from higher to lower energy levels result in energy being emitted (given out). Electron transitions from lower to higher energy levels result in energy being absorbed (taken in). The correct answer is 'electron transitions take place from a higher to a lower electronic energy level' since it is the only answer that will result in energy being emitted (to give an emission spectrum) rather than absorbed.
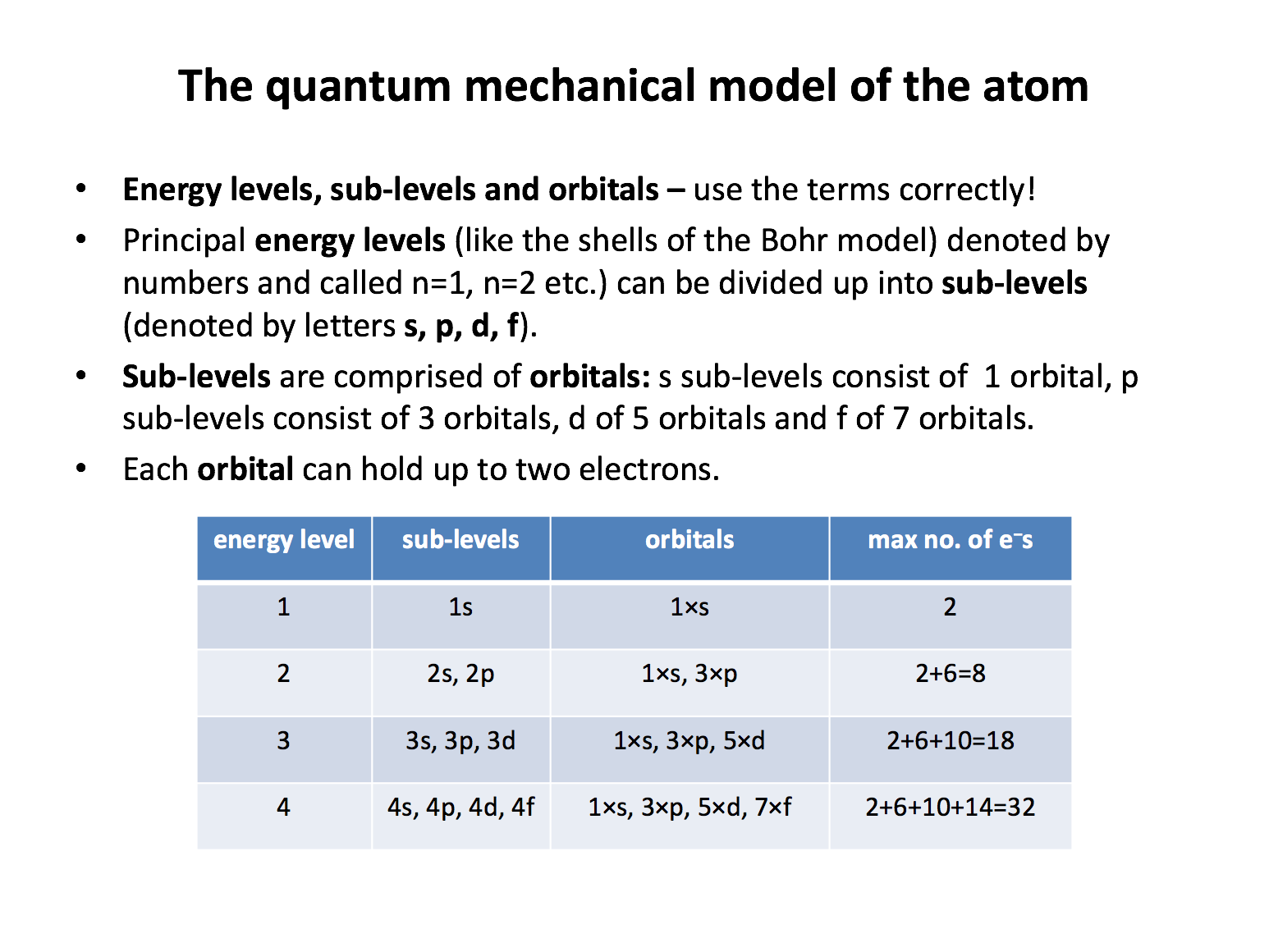
What is the maximum number of electrons that can be held the n=3 electronic energy level in an atom?
Each and every orbital can hold a maximum of two electrons. Any s sub-level contains one orbital and can therefore hold two electrons. Any p sub-level contains three orbitals and can therefore hold six electrons. Any d sub-level contains five orbitals and can therefore hold ten electrons. The n=3 energy level consists of the 3s, 3p and 3d sub-levels. Thus the answer is 18: 2 (3s) + 6 (3p) + 10 (3d). The 4s sub-level is filled before the 3d sub-level, which may cause some confusion here, but the 3d sub-level is still part of the n=3 energy level.
What is the maximum number of electrons that can be held in an orbital, a p sub-level, and the n=2 energy level respectively?
Each and every orbital can hold two electrons. Any s sub-level contains one orbital and can therefore hold two electrons. Any p sub-level contains three orbitals and can therefore hold six electrons. Any d sub-level contains five orbitals and can therefore hold ten electrons. The n=2 energy level consists of the 2s and 2p sub-levels. Thus the answer is 2, 6, 8: A maximum of 2 electrons can be held in an orbital, 6 in a p sub-level, and 8 in the n=2 energy level (2s and 2p; 2+6=8).
Which of the following provide evidence for the existence of electrons in discrete energy levels?
1: The line emission spectrum of hydrogen
2: Electron spin
3: The mass spectrum of hydrogen
The line emission spectrum of hydrogen is composed of discrete lines that represent particular frequencies of light. Only certain frequencies are produced by electron transitions from higher to lower energy levels, suggesting that electrons only exist at certain energies within the atom. Thus the line emission spectrum provides evidence for the existence of electrons in discrete energy levels. Electron spin and the mass spectrum of hydrogen do not provide any evidence for this model.
Paper 1
Core (SL&HL): Atomic Structure core (SL and HL) paper 1 questions
AHL (HL only): Atomic Structure AHL (HL only) paper 1 questions
Paper 2
Core (SL&HL): Atomic Structure core (SL & HL) paper 2 questions
AHL (HL only): Atomic Structure AHL (HL only) paper 2 questions
How much of Electron configuration have you understood?










 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn