Topics 16.1 and 16.2
Paper 1 style questions are multiple choice. You are not permitted to use a calculator or the data book for these questions, but you should use a periodic table.
A periodic table pop-up is available on the left hand menu.
What are the units of the rate constant for a third order reaction?
Units of the rate constant can be worked out by cancelling out in the equation in the same way as calculating the numerical value for the rate constant:
e.g. Rate = k[Y]3
k = Rate / [Y]3
units = mol dm–3 s–1 / (mol dm–3)(mol dm–3)(mol dm–3)
So cancel one moles per decimetre cubed from top and bottom:
units = s–1 / (mol dm–3)(mol dm–3)
Mulitiply out brackets
units = s–1 / mol2 dm−6
Move units from bottom to top and therefore invert the sign:
mol–2 dm6 s–1 is the correct answer.
Or they can be learnt:
Overall order of reaction | 1st | 2nd | 3rd | 4th |
Rate constant units | s–1 | mol–1 dm3 s–1 | mol–2 dm6 s–1 | mol–3 dm9 s–1 |
Which gives the value of \( ln A\) when a graph of \( ln k\) (y-axis) is plotted against \(1 \over T\)(x-axis)?
\(ln k = {-E_a \over RT} + ln A\)
The equation given in the question can be compared to the equation for a straight line \(y = mx + c\):
\(ln k = {-E_a \over R}.{1 \over T} + ln A\)
The temperature term has been separated from the activation energy term, and this makes it easier to see that if y = lnk and x = 1/T then the plot will generate a straight line, and the gradient, m, will be –Ea/R and the intercept, c, will be lnA.
So the correct answer is Intercept on the y-axis.
What is the rate equation for the reaction mechanism below?
Step 1 (fast step): 2A(g) → B(g) + C(g)
Step 2 (slow step): B(g) + D(g) → E(g) + F(g)
Only reactants that are in the rate determining step – slowest step – (or contribute to an intermediate that is in the rate determining step) can appear in the rate equation and therefore have an effect upon rate. One mole of B and D appear in the slow/rate determining step and thus [D] has an order of 1. B, however, is an intermediate (since it is made in step 1 and used up in step 2). Two moles of A are used to make the intermediate B, and therefore [A] has an order of 2.
In a sense, the rate equation that looks like it should be Rate = k[B][D] becomes Rate = k[A]2[D], since intermediates cannot appear in the rate equation.
Rate = k[A]2[D] is the correct answer.
The following data was obtained for the chemical reaction: X + Y → Products
| [X] (mol dm–3) | [Y] (mol dm–3 ) | Initial Rate (mol dm–3 s–1) |
| 0.20 | 0.10 | 2.0 × 10–3 |
| 0.20 | 0.30 | 1.8 × 10–2 |
| 0.40 | 0.10 | 4.0 × 10–3 |
What is the order of reaction with respect to [X] and the order of reaction with respect to [Y]?
The correct answer is 1st with respect to [X] and 2nd with respect to [Y].
When the concentration of Y is tripled (experiments 1 to 2) the rate increases nine fold (0.002 to 0.018 – make sure you understand standard form; to a positive power of ten moves the decimal place that many places to the right, negative power to the left). [Y] × 3 causes rate × 9, and 32=9, so [Y] is second order.
When the concentration of X is doubled (experiments 1 to 3) the rate also doubles (0.002 to 0.004). [X] × 2 causes rate × 2, and 21=2, so [X] is first order.
The following data was obtained for the chemical reaction: Y + Z → Products
| [Y] (mol dm–3) | [Z] (mol dm–3 ) | Initial Rate (mol dm–3 s–1 ×10−2) |
| 0.20 | 0.20 | 0.22 |
| 0.40 | 0.20 | 0.88 |
| 0.40 | 0.40 | 0.88 |
What is the overall order of reaction?
When the concentration of only Y is doubled (experiments 1 to 2) the rate is quadrupled. [Y] × 2 causes rate × 4, so [Y] is second order. When the concentration of only Z is doubled (experiments 2 to 3) the rate doesn't change, so [Z] is zero order. Thus [Y] is 2nd order and [Z] is zero order. Adding the orders gives 2nd order (or order 2) overall.
So the correct answer is 2nd.
What can be deduced from this graph?
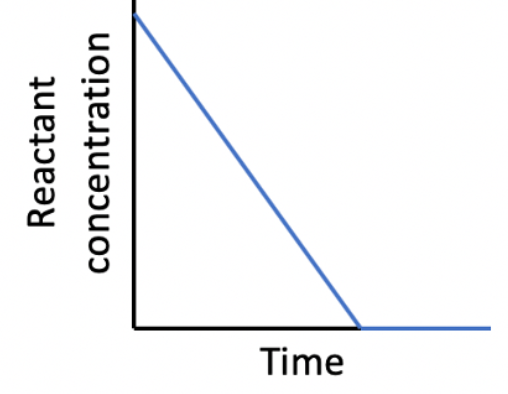
The relationships between reactant concentration and time are shown below:

A straight line (decreasing concentration does not impact rate) indicates zero order.
A curved line with a constant half-life indicates first order.
A curved line with a half-life that is not constant indicates second order.
The reaction is zero order with respect to the reactant is therefore the correct answer.
The rate expression for a reaction is Rate = k[A]2[B]
By which factor will the rate of reaction increase if [A] and [B] are both increased by a factor of 3?
When the concentration of A is tripled the rate increases nine fold: [A] × 3 causes rate × 9, 32 = 9.
When the concentration of B is tripled the rate is also tripled: [B] × 3 causes rate × 3, and 31 = 3.
Rate × 9 and rate × 3 gives a total (9×3) of × 27.
So 27 is the correct answer.
The mechanism below represents the SN1 reaction between 2-bromo-2-methyl propane and a hydroxide ion:
Step 1 (slow step): (CH3)3CBr → (CH3)3C+ + Br–
Step 2 (fast step): (CH3)3C+ + OH– → (CH3)3COH
The hydroxide ion does not take part in step 1 and the bromide ion does not take part in step 2.
Which of the following is correct?
This is a two step reaction with the first (slowest) step being the rate determining step (RDS).
The RDS has only the halogenoalkane species as a reactant (it is unimolecular) so the rate expression will be rate=k[(CH3)3CBr]. That is first order with respect to (CH3)3CBr, and first order overall.
Molecularity is the number of particles that react together in a given reaction/step. In step 1 the only reactant is (CH3)3CBr so the molecularity is 1 (a unimolecular step). (In step 2 the reactants are (CH3)3C+ and OH– so the molecularity is 2 (a bimolecular step).
The molecularity of the rate determining step is 1 is the correct answer.
What is the equation for the overall reaction given the reaction mechanism below?
Step 1 (fast step): A + B → C + 2D
Step 2 (fast step): D + B → E + F
Step 3 (slow step): F + D + C → H
Intermediates need to be cancelled. That is, if a species appears as a product in one step and then as a reactant in another step, it should be cancelled from the equation:
Step 1 (fast step): A + B → C + 2D
Step 2 (fast step): D + B → E + F
Step 3 (slow step): F + D + C → H
This leaves only the non-highlighted species, so A + 2B → E + H is the correct answer.
Which of the following represents a concentration vs rate graph for a zero order reactant?
The relationships between reactant concentration and rate are shown below:
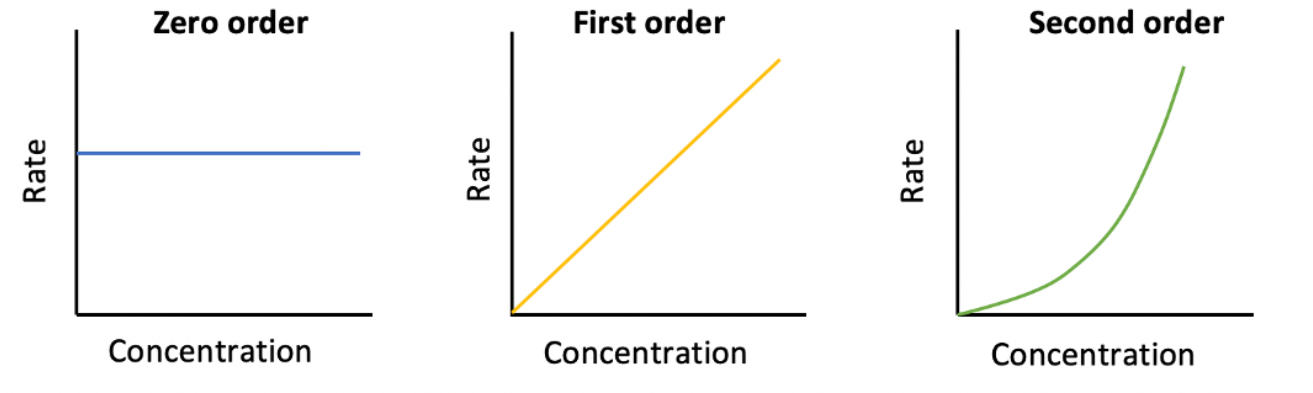
A straight horizontal line indicates zero order.
A straight line indicates first order.
A curved line indicates second order.
The fourth graph with a straight line sloping down indicates the relationship between time and reactant concentration, seen here:

Therefore the correct answer is
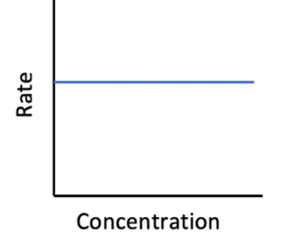
How much of Kinetics AHL (HL only) paper 1 questions have you understood?


 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn