Topics 18.1 to 18.3
Paper 1 style questions are multiple choice. You are not permitted to use a calculator or the data book for these questions, but you should use a periodic table.
A periodic table pop-up is available on the left hand menu.
Which combination will produce an alkaline buffer solution in water?
An alkaline buffer solution is the solution of a weak base and the salt of the weak base (that resists/opposes changes in pH upon addition of small amounts of acid or base).
0.20 mol NH3 and 0.05 mol H2SO4 will produce an alkaline buffer, since 0.05 mol H2SO4 will neutralise half (0.10 mol) of the NH3 (1:2 ratio), leaving unreacted weak base, NH3, and the salt of the weak base, (NH4)2SO4.
2NH3 + H2SO4 → (NH4)2SO4
0.20 mol NH3 and 0.05 mol H2SO4 is therefore the correct answer.
Incorrect answers
0.20 mol NH3 and 0.10 mol H2SO4 will result in all of the ammonia, NH3, being neutralised (1:2 ratio) so this cannot form a buffer.
A combination of ethanoic acid, CH3COOH, and sodium ethanoate, CH3COONa, (in any reasonable quantities) will form a buffer solution, but that will be an acidic buffer.
A combination of an excess of ethanoic acid with sodium hydroxide will also form a buffer solution, since all of the sodium hydroxide will be neutralised and will produce sodium ethanoate, but an excess of NaOH will neutralise all the acid and the solution will not be a buffer (as is the case in this question).
Which of the following could correctly be described as a nucleophile?
A nucleophile is a substance which can donate a lone pair of electrons, which is an identical description to that of a Lewis base.
Likewise, an electrophile is equivalent to a Lewis acid as it can accept a lone pair of electrons.
Lewis base is therefore the correct answer.
Which compound is alkaline when dissolved in water?
CH3COOH is an acid.
The other three options are all salts.
As a useful rule-of-thumb, salts formed from strong acids and strong bases (e.g. NaCl) and those from weak acids and weak bases give approximately neutral solutions of pH=7.
Salts formed from strong acids and weak bases (e.g. NH4Cl) are acidic and those from weak acids and strong bases (e.g. CH3COONa) are alkaline.
Salt hydrolysis, is due to the reaction of the conjugate bases/acids (salt ions) of weak acids/bases with water:
E.g. NH4+ + H2O ⇌ NH4OH + H+
E.g. CH3COO– + H2O ⇌ CH3COOH + OH–
CH3CO2Na is therefore the correct answer.
Which can act as a Lewis acid, but not a Bronsted-Lowry acid?
A Lewis acid is a substance that can accept a pair of electrons from a Lewis base, forming a co-ordinate (dative) bond.
A Bronsted-Lowry acid can donate an H+ ion.
Boron in BF3 is electron deficient (it has an incomplete octet) and so it can accept a pair of electrons. BF3 cannot act as a Bronsted-Lowry acid, as it has no hydrogens.
None of the other species can act as Lewis acids, as none of the atoms are electron deficient, and they all have complete octets (and cannot expand them); or two electrons in the case of hydrogen.
The correct answer is therefore BF3.
Complete this question without a calculator:
Which solution, of equal concentrations, will have the lowest pH?
Weak acids only partially dissociate: HA ⇌ H+ + A−
\(K_a = {{ [A^-][H^+] } \over [HA]}\)
Therefore the Ka for a stronger acid will be greater than for a weaker acid (more ions on top of fraction).
pKa = −log10Ka
Therefore higher Ka leads to lower pKa (similarly to the relationship between [H+] and the pH scale).
Stronger acids will always have lower pH than weak acids of the same concentration because of the increased dissociation.
Without a calculator we need to remember that pKa is a logarithmic scale, so Ka = 1.4 × 10−3 will give a pKa of approx. 3 (the × 10−3 tells us this) and Ka = 1.0 × 10−10 will give a pKa of approx.10.
The strongest acid (with a pKa of approx. 3) is therefore CH2ClCOOH, and so this will have the lowest pH.
Therefore CH2ClCOOH (Ka = 1.4 × 10−3) is the correct answer.
The graph below represents a pH titration curve. Which statements are correct?
1: The pH curve could have been produced by titration of ethanoic acid with sodium hydroxide solution.
2: The equivalence point is at pH 7.
3: The buffer region includes the half-equivalence point (12.5cm3 added).
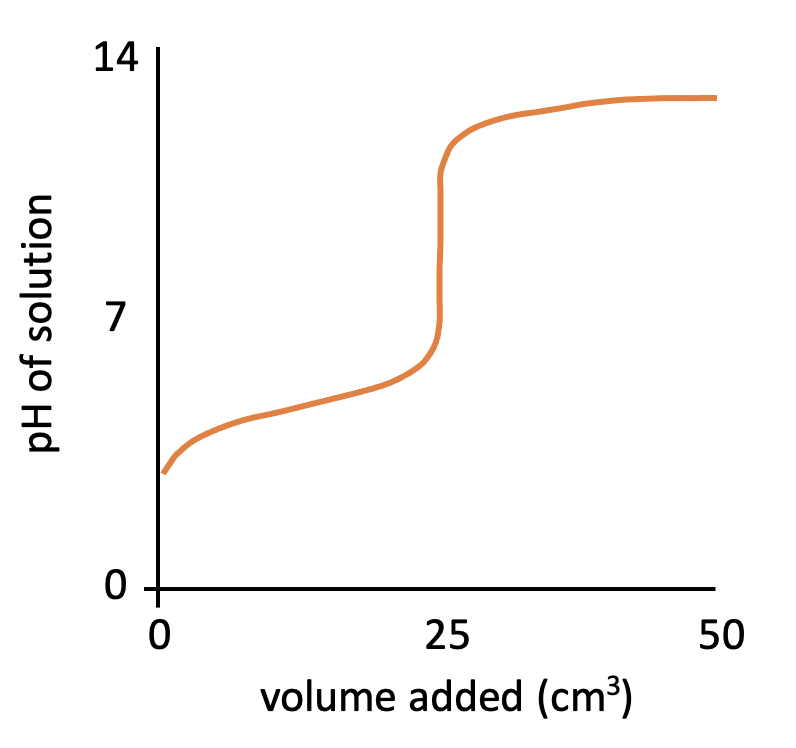
The shapes of the four pH titration curves need to be learned.
The starting pH (∼3), high finishing pH and the steep inflection in the curve above pH 7 indicate that this is a weak acid, strong base pH curve. Therefore the curve could have been produced by titration of ethanoic acid with sodium hydroxide solution.
The equivalence point is the point at which the moles of acid = moles of base, and for a weak acid and strong base pH curve, this is typically around pH 9 (not pH 7).
At half the equivalence point (at 12.5cm3) half of the acid has been neutralised so the concentration of acid and salt is the same: [HA] = [A–] which means this is a buffer solution, and so this part of the curve is known as the buffer region.
1 and 3 only is therefore the correct answer.
What changes occur in water as temperature increases (Kw = 1 × 10−14 at 298K)?
H2O (l) ⇌ H+ (aq) + OH− (aq) ΔH = +56 kJ
If the temperature is increased the equation will shift in the endothermic direction, which means the equilibrium will shift to the right.
Shifting the equilibrium to the right will increase the quantity of H+ ions and OH− ions in the aqueous solution; [H+] and [OH−] will increase.
Kw = [H+] × [OH−]
Kw will therefore increase as the concentration of H+ and OH− ions increases, and pH will decrease as H+ ion concentration increases, since pH=−log10[H+].
Kw will increase and the pH will decrease is therefore the correct answer.
An indicator, HIn, has pKa = 4.2.
HIn (aq) ⇌ H+ (aq) + In− (aq)
HIn (aq) is yellow, and In− (aq) is blue.
Which statement/s are correct?
1: At pH 7.0 the indicator would be yellow
2: At pH 4.2 the indicator would be green
3: At pH 4.2, [Hin] = [In−]
Indicators are weak acids that change colour when [HIn] = [In−] (In practice the colour changes across a pH range). At the point when [HIn] = [In−], Ka = [H+] and pH = pKa (see expression below; [HIn] and [In−] cancel each other out).
\(K_a = {{[H^+][In^-]} \over [HIn]}\)
Therefore indicators change colour across a range 'straddling' pH = pKa.
Therefore, for the indicator in this question, at pH 4.2, [Hin] = [In−] and since the protonated and deprotonated forms are yellow and blue, a 50:50 mixture will be green.
At pH 7.0 the indicator in this question will be blue (not yellow), since less H+ will push the equilibrium right and [Hin] << [In−]
2 and 3 only is therefore the correct answer.
Which combination of acid and base is most likely to have a pH of 5.5 at the equivalence point in a titration?
The shapes of the four pH titration curves need to be learned.
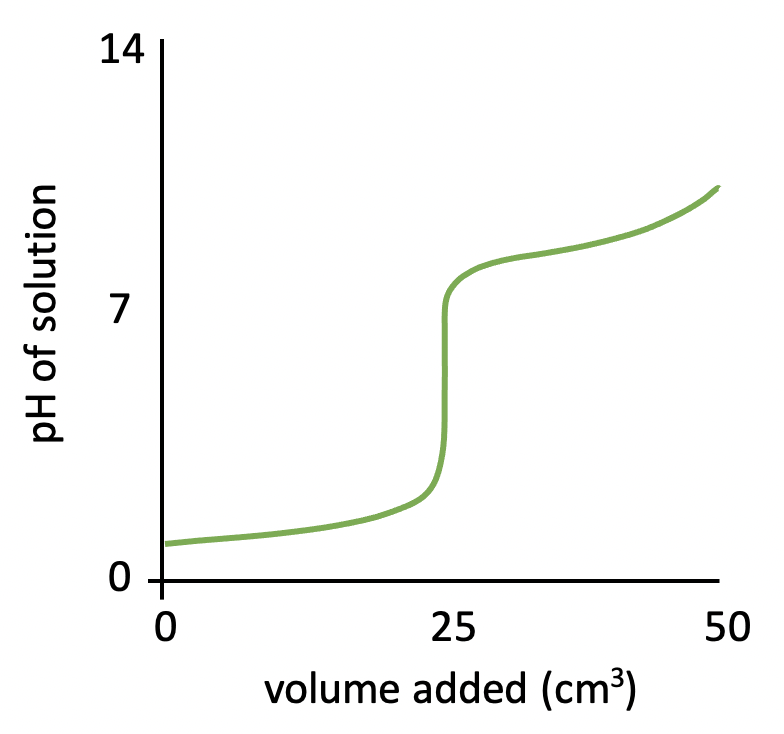
This is a strong acid, weak base pH curve. Notice the low starting pH, finishing pH of ∼11, and the steep inflection in the curve below pH 7.
The equivalence point is at pH 5/6.
Hydrochloric acid and ammonia solution (strong acid and weak base) is therefore the correct answer.
Complete this question without a calculator: A buffer solution can be produced by mixing ethanoic acid, CH3COOH (aq), and sodium hydroxide, NaOH (aq).
If 25.0 cm3 of 0.100 mol dm−3 CH3COOH (aq) is used, what volume of 0.100 mol dm−3 NaOH (aq) will produce a buffer, and what will the pH of the buffer be?
A combination of ethanoic acid, CH3COOH, and the salt of the weak acid e.g. sodium ethanoate, CH3COONa, (in any reasonable quantities) will form a buffer solution, and that will be an acidic buffer (with a pH < 7).
A combination of an excess of ethanoic acid with sodium hydroxide will also form a buffer solution, since all of the sodium hydroxide will be neutralised and will produce sodium ethanoate (CH3COOH + NaOH → CH3COONa + H2O), and this results in a mixture of unreacted ethanoic acid and sodium ethanoate.
12.5 cm3 of NaOH and pH = 4.8 is therefore the correct answer, since this volume of NaOH will neutralise half of the ethanoic acid, and the resulting buffer will be acidic (pH < 7).
How much of Acids and Bases AHL (HL only) paper 1 questions have you understood?


 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn