 There is a great deal of content here; lots of information that must be learned, with many links to other parts of the course. If it is too daunting, perhaps come back to it after completing other sections. Periodicity can pull together your understanding towards the end of the revision period. Periodicity refers to the pattern in the change in the properties of elements across a period. The position of an element in the periodic table allows us to make predictions about its physical and chemical properties. The fundamental principles covered in this topic underpin much chemistry.
There is a great deal of content here; lots of information that must be learned, with many links to other parts of the course. If it is too daunting, perhaps come back to it after completing other sections. Periodicity can pull together your understanding towards the end of the revision period. Periodicity refers to the pattern in the change in the properties of elements across a period. The position of an element in the periodic table allows us to make predictions about its physical and chemical properties. The fundamental principles covered in this topic underpin much chemistry.
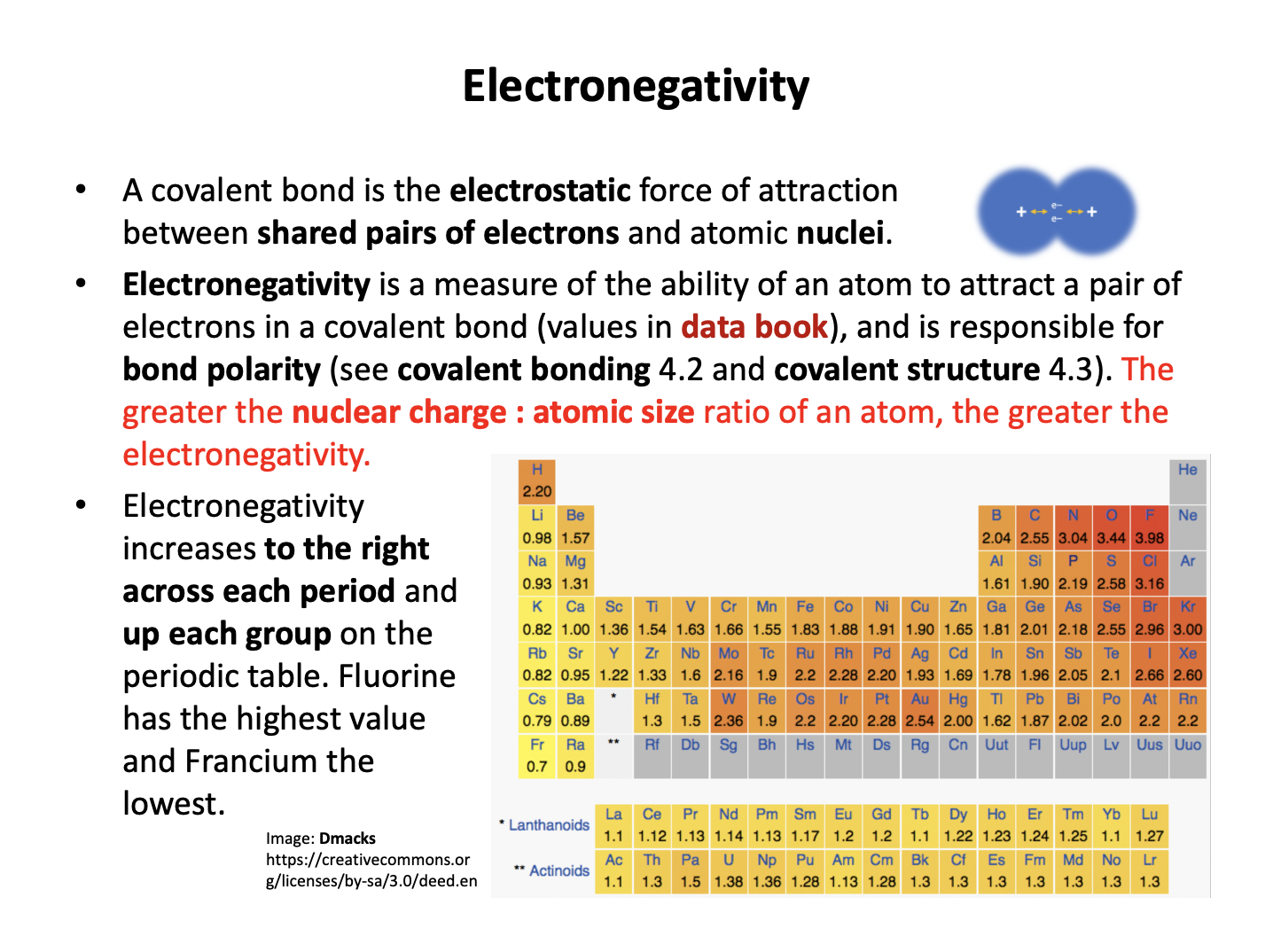
Terminology is important, so learn the vocabulary thoroughly using the flash cards. Electrostatic attractions between the protons in the nucleus and the electrons in the outer/valence shell form the basis for many periodic trends: the force of attraction increases as the magnitude of the charge increases and decreases with distance between the charges. You should be able to explain each of the trends covered here across periods and down groups.
Ensure you are confident using the terms below and learn the asterisked* definitions
nuclear charge, shielding*, atomic radius, first ionisation energy*, second ionisation energy*, first electron affinity*, second electron affinity*, electronegativity*, properties of metals, properties of non-metals, metalloids, amphoteric, displacement
Which of the following would be correctly included in the definition of first ionisation enthalpy?
1: One mole of 1+ ions are formed...
2: ...from the element...
3: ...in its standard state.
First ionisation enthalpy is a definition that needs to be learned:
First ionisation enthalpy is the enthalpy change when one electron is removed from each atom in one mole of gaseous atoms.
Thus 1 only is the correct answer.
One mole of 1+ ions are formed, but first ionisation enthalpy involves gaseous atoms (not the element in its standard state)
Which of the following would be correctly included in an explanantion of why first ionisation enthalpy generally increases across period 3 of the periodic table?
1: Nuclear charge increases
2: Electrons are being added to the same energy level
3: Electron-electron repulsion is increasingly greater than the attraction between the outer electrons and the nucleus
First ionisation enthalpy is a definition that needs to be learned:
First ionisation enthalpy is the enthalpy change when one electron is removed from each atom in one mole of gaseous atoms.
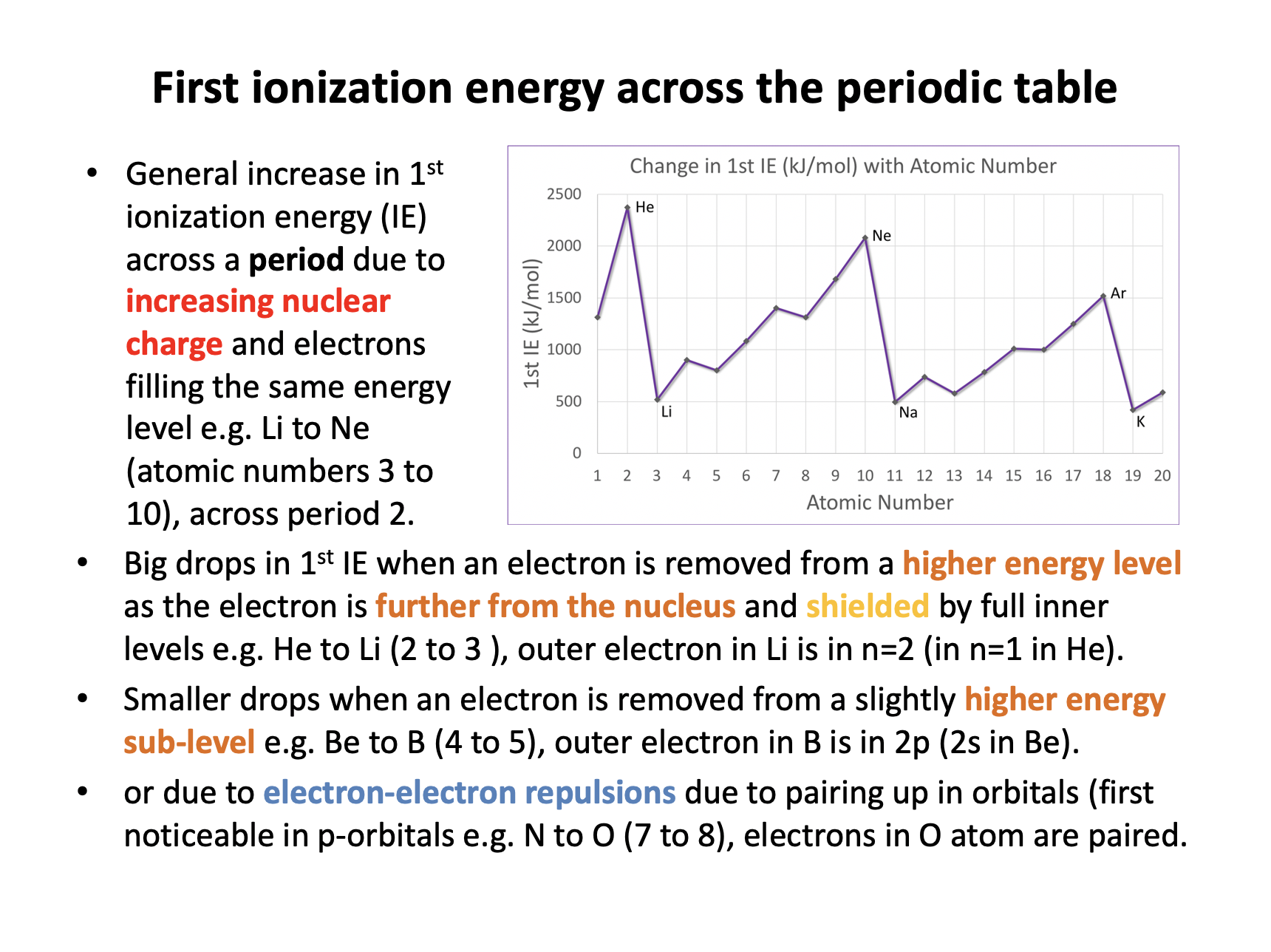
Across period 3 there is a general increase in 1st ionization energy (IE) due to increasing nuclear charge and electrons filling the same energy level; that is filling the 3s and 3p sub-levels. The electrons being in the same energy level are 'initially' no further form the nucleus, but the nuclear charge increases, increasing the attraction between the nucleus and the electrons. Overall the attraction between the outer electrons and the nucleus outweighs the increasing electron-electron repulsion, so statement 3 is incorrect.
Thus 1 and 2 only is the correct answer.
Which of the following contribute to oxygen (1314 kJmol−1) having a lower first ionisation energy than nitrogen (1402 kJmol−1), even though oxygen has a higher nuclear charge than nitrogen?
1 The electron removed is in a higher energy level, with less attraction to the nucleus
2 The electron removed is paired-up with another electron in the same orbital, resulting in repulsion
3 The electron removed has greater 'shielding' (repulsion) by complete inner energy levels of electrons
The electron removed from oxygen is removed from the 2p sub-level. The electron removed from nitrogen is also in the 2p sub-level. Thus explanations 1 and 3 do not contribute. Oxygen's outer electron is however, paired-up, unlike the electrons of nitrogen which occupy orbitals singly (on their own) so explanation 2 does apply.
Which of the following contribute to potassium (419 kJmol−1) having a lower first ionisation energy than argon (1520 kJmol−1), even though potassium has a higher nuclear charge than argon?
1 The electron removed is in a higher energy level, with less attraction to the nucleus
2 The electron removed is paired-up with another electron in the same orbital, resulting in repulsion
3 The electron removed has greater 'shielding' (repulsion) by complete inner energy levels of electrons
The electron removed from potassium is removed from the 4s sub-level. The electron removed from argon is from the 3p sub-level. Explanations 1 and 3 both contribute to the ionisation energy of potassium being lower than argon; the 4s sub-level is in the n=4 energy level, further from the nucleus with less nuclear attraction and the inner complete levels of electrons will shield (repel) the outer electron to a greater extent (than in argon). Potassium's outer electron in 4s is, however, not paired-up, so explanation 2 does not apply.
Which of the equations below represents the first electron affinity of oxygen?
First electron affinity and second electron affinity are definitions that need to be learned:
First electron affinity is the enthalpy change when one electron is gained by each atom in one mole of gaseous atoms.
Second electron affinity is the enthalpy change when one electron is gained by each 1− ion in one mole of gaseous ions.
Thus O(g) + e− → O−(g) is the correct answer, as this is the first electron affinity of oxygen. Equations involving oxygen molecules (O2) are incorrect.
Which of the following would be correctly included as part of an explanantion of why atomic radii generally decrease across period 3 of the periodic table?
1: Nuclear charge increases
2: The number of electrons increases
3: Electrons are being added to the same energy level
Across period 3 there is a general decrease in atomic radii due to increasing nuclear charge and electrons filling the same energy level; that is filling the 3s and 3p sub-levels.
The electrons are in the same energy level, but the nuclear charge increases, increasing the attraction between the nucleus and the electrons and pulling them in more tighly/closer to the nucleus. Overall the attraction between the outer electrons and the nucleus outweighs the increasing electron-electron repulsion.
The number of electrons increasing is incidental; increased electron-electron repulsion would cause an increase in atomic size, but here it is outweighed by the increased attraction.
Thus 1 and 3 only is the correct answer.
Which of the following statements is true with regard to group 1?
1: The atomic radius of sodium is greater than that of potassium
2: The atomic radii get smaller going down the group
3: Caesium has a greater number of energy levels filled with electrons than rubidium
Down any group of the periodic table there is a general increase in atomic radii due to the electrons filling new energy levels.
The nuclear charge increases, but because the electrons are being added to energy levels that are further form the nucleus, there is increased shielding (repulsion by inner electrons) decreasing the attraction between the nucleus and the electrons and pulling them in less tighly to the nucleus. Overall the attraction between the outer electrons and the nucleus is weaker than the increasing electron-electron repulsion due to shielding.
Statements 1 and 2 here are therefore false as they state the opposite trend. Statement 3 is true.
Thus 3 only is the correct answer.
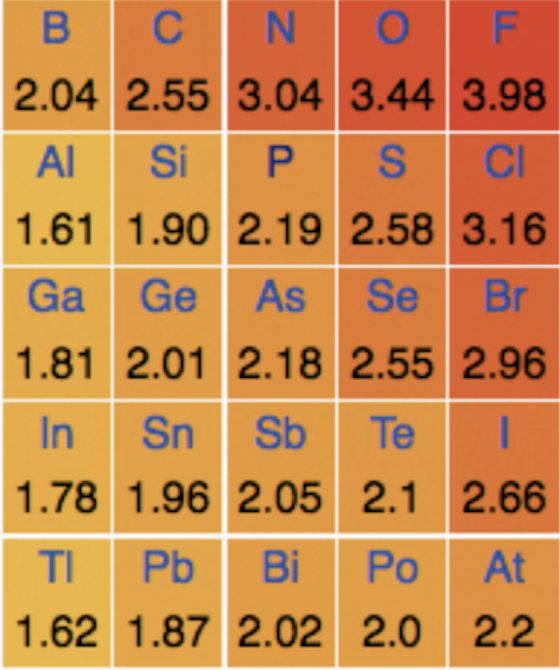
Which of the following statements is/are true with regard to group 17?
1: The atomic radius of bromine is greater than that of chlorine
2: The ionic radius of chlorine is greater than its atomic radius
3: The electronegativity of chlorine is greater than bromine
Down any group of the periodic table there is a general increase in atomic radii due to the electrons filling new energy levels.
The nuclear charge increases, but because the electrons are being added to energy levels that are further from the nucleus, there is increased shielding (repulsion by inner electrons) decreasing the attraction between the nucleus and the electrons and pulling them in less tighly to the nucleus. Overall the attraction between the outer electrons and the nucleus is weaker than the increasing electron-electron repulsion due to shielding. Statements 1 is therefore true.
Anions (negative ions) like Cl– are always larger than the corresponding atom (and cations are always smaller) as increased number of electrons increases electron-electron repulsion, so statement 2 is also true.
Statement 3 is also true as electronegativity increases to the right and upwards on the periodic table (increasing up a group), so chlorine does have a greater electronegativity than bromine.
Thus 1, 2 and 3 is the correct answer.
Which of the following statements is/are true with regard to metals?
1: Metals tend to form acidic oxides.
2: Metals tend to be malleable and ductile
3: Metals tend to be better at losing electrons than non-metals
Metals tend to form basic (alkaline) oxides, not acidic oxides, so statement 1 is false.
Metals' outer electrons are delocalised and they have giant structures. Metals are often: good conductors of electricity and heat; high in melting and boiling point; malleable; ductile; sonorous; lustrous (shiny). Therefore statement 2 is true.
Metals have low electronegativity and tend to lose electrons when they react. Therefore statement 3 is true (as non-metals tend to gain electrons).
Thus 2 and 3 only is the correct answer.
Which of the following statements is/are true with regard to the boiling points of elements across period 3?
1: The boiling points increase from sodium to aluminium due to an increase in metallic bond strength
2: Silicon has a low boiling point because it has a simple molecular structure
3: The boiling points decrease from sulfur to argon due to a decrease in intermolecular force strength
Metals (Na, Mg , Al) have high mp/bp; strong metallic bonds in giant structures are broken. Higher ionic charge and smaller ions tend to have higher mp/bp due to stronger metallic bonds. Statement 1 is true.
Non-metals or metalloids with giant structures (Si) have high mp/bp; strong covalent bonds are broken. So statement 2 is false as silicon does not have a low boiling point or a simple molecular structure.
Non-metals with simple molecular structures (S8, Cl2) or gaseous atoms (Ar) have low mp/bp; only intermolecular forces need to be broken. Different intermolecular forces vary in strength, but are all relatively weak. Statement 3 is true - the molecules get smaller and so have weaker London dispersion forces between them.
Thus 1 and 3 only is the correct answer.
Which statement about the melting points down groups 1 (alkali metals) and 17 (halogens) is correct?
Group 1 metals have metallic bonding and therefore giant structures. Melting points are relatively low for metals as the atoms are large and form 1+ ions so the attraction between the nucleus and ‘sea of electrons’ is not as strong as in other metals. Nonetheless strong metallic bonds must be broken. The melting points (values in data book) become lower down the group because of the increasing atomic/ion size leads to less attraction..
Group 17 non-metals; the halogens, have covalent bonding and simple molecular structures. The atoms get bigger down the group, so the diatomic halogen molecules get bigger down the group. These non-polar molecules have weak London dispersion forces that must be broken. The melting points (data book) become higher down the group as London dispersion forces get stronger as the number of electrons/molar mass of a molecule increases.
Thus, The melting points decrease down group 1 and increase down group 17 is the correct answer.
Which of the following statements is/are true with respect to aluminium?
1: The boiling point of aluminium is high
2: Aluminium forms only basic oxides
3: Aluminium oxide (Al2O3) is amphoteric
Metals have high mp/bp; strong metallic bonds in giant structures are broken. Statement 1 is true.
We would normally expect metals to form basic oxides, but we need to remember that aluminium oxide is amphoteric, which means it can react as an acid or base. Therefore statement 3 is true, but statement 2 is false.
Thus 1 and 3 only is the correct answer.
Which of the following displacement reactions involving group 17 elements could occur as written?
1: Cl2 + 2NaF → F2 + 2NaCl
2: Br2 + 2NaCl → Cl2 + 2NaBr
3: Br2 + 2NaI → I2 + 2NaBr
Halogens decrease in reactivity going down the group and this is explained in terms of the decreasing attraction between outer electrons and the nucleus as the atoms become larger. The outer electron is less easily gained as it is further from the nucleus and has increased ‘shielding’ from inner energy levels, so there is weaker electron-nucleus electrostatic attraction.
A more reactive halogen will displace a less reactive halogen from its compound. (A 'higher in the group' halogen will displace a lower halogen.) Therefore 1 and 2 won't react as chlorine is less reactive than flourine and bromine is less reactive than chlorine, but reaction 3 will react as written.
Thus 3 only is the correct answer.
Paper 1
Core (SL&HL): Periodicity core (SL and HL) paper 1 questions
AHL (HL only): Periodicity AHL (HL only) paper 1 questions
Paper 2
Core (SL&HL): Periodicity core (SL & HL) paper 2 questions
AHL (HL only): Periodicity AHL (HL only) paper 2 questions
How much of Periodic trends have you understood?


























 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn