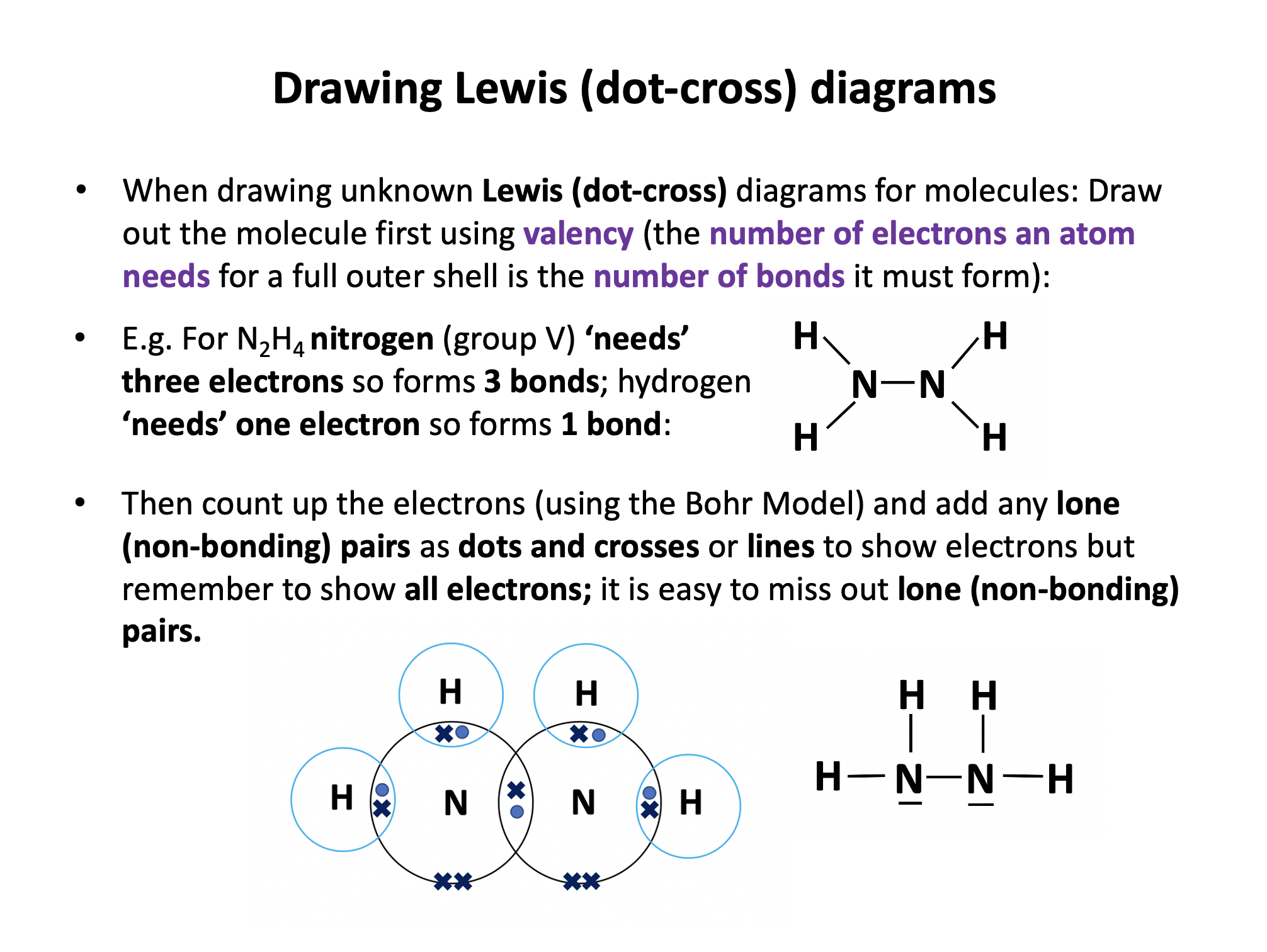
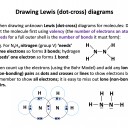
 Our model of covalent bonding and structure is based on the Bohr model of the atom and represented with Lewis diagrams. Lewis diagrams can be drawn with dots and crosses or with lines that represent a pair of outer-shell (valence) electrons.. It is important to become familiar with both notations; dots and/or crosses, or lines, so these notations are used interchangeably throughout this website. Always check that you have the correct number of electrons in total after you've drawn a covalent molecule; it is easy to slip up here!
Our model of covalent bonding and structure is based on the Bohr model of the atom and represented with Lewis diagrams. Lewis diagrams can be drawn with dots and crosses or with lines that represent a pair of outer-shell (valence) electrons.. It is important to become familiar with both notations; dots and/or crosses, or lines, so these notations are used interchangeably throughout this website. Always check that you have the correct number of electrons in total after you've drawn a covalent molecule; it is easy to slip up here!
Ensure you are confident using the terms below and learn the asterisked* definitions
a polar bond*, a dative (coordinate) bond*, VSEPR theory*, a polar molecule, the octet rule, expanded octet, incomplete octet, valency, resonance structures, delocalised electrons, allotropes, lattice (or giant) structure.
Which of the following are included in the rules of VSEPR (Valence Shell Electron Pair Repulsion) theory?
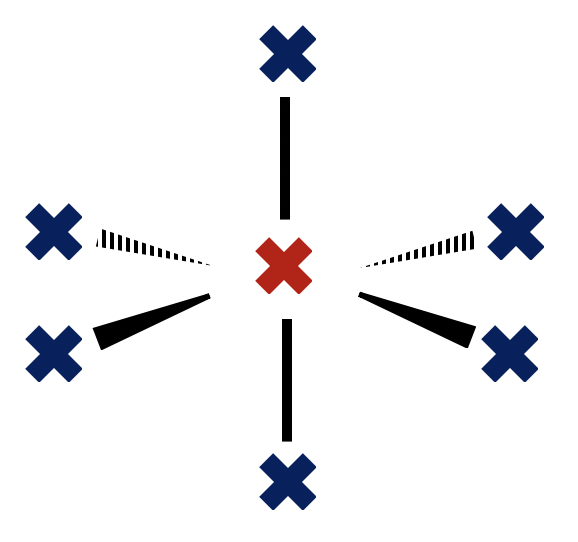
1: The repulsion exhibited by a lone pair of electrons on a bonding pair is greater than the repulsion exhibited by a bonding pair on another bonding pair.
2: Electron domains will repel as far apart as possible (in 3D space) around a central atom.
3: The repulsion exhibited by a lone pair of electrons on another lone pair is less than the repulsion exhibited by a bonding pair on another bonding pair.
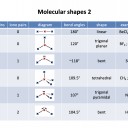
Draw a Lewis (dot-cross) diagram for silane (SiH4). What is the shape around the central silicon atom and what are the likely bond angles?
Silane is the same shape as methane (CH4); tetrahedral, with bond angles of 109.5°. The Lewis diagram and shape are drawn below. Remember that molecules are three-dimensional, even though we draw the Lewis diagrams in two dimensions on the page.
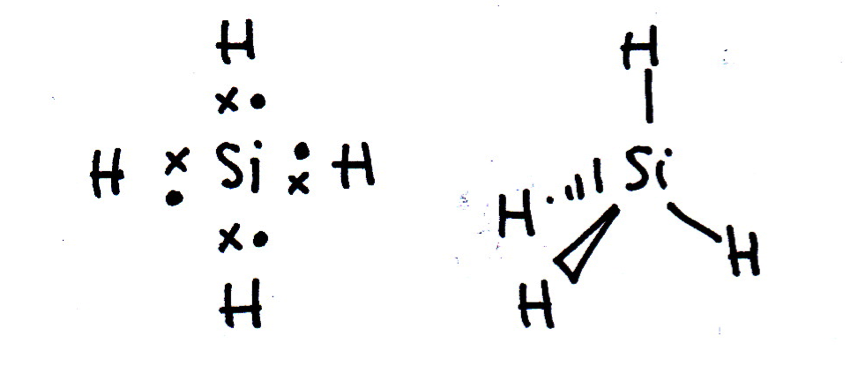
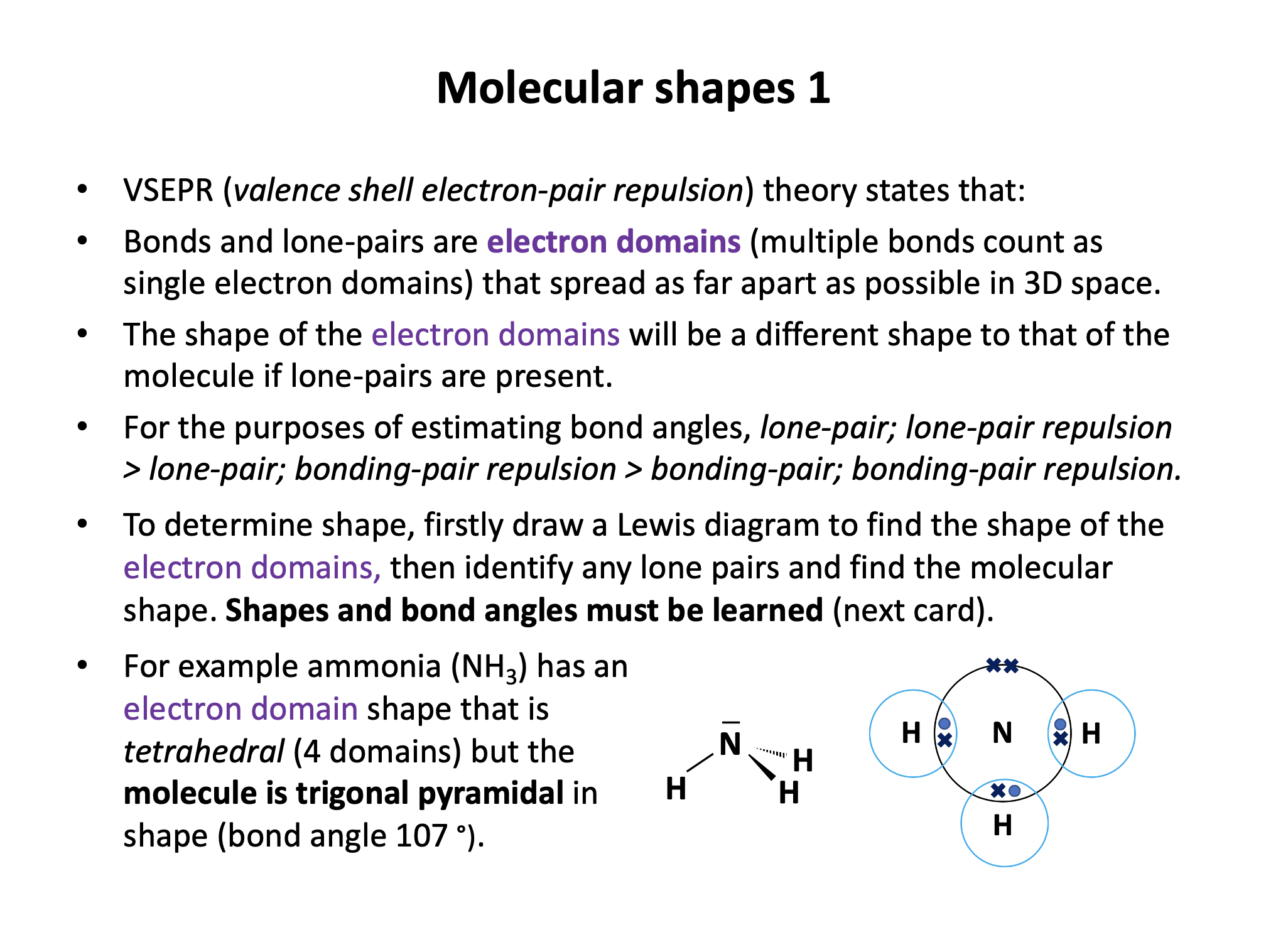
Draw a Lewis (dot-cross) diagram for phosphine (PH3). What is the shape around the central phosphorus atom and what are the likely bond angles?
Phosphine is the same shape as ammonia (NH3); trigonal pyramidal, with bond angles that might be estimated at approx 107° (although they are lower). The electron domains form a tetrahedral shape, but one bond is missing (it is a lone pair).
The Lewis diagram and shape are drawn below. Remember that molecules are three-dimensional, even though we draw the Lewis diagrams in two dimensions on the page.

Draw a Lewis (dot-cross) diagram for hydrogen sulfide (H2S). What is the shape around the central sulfur atom and what are the likely bond angles?
Hydrogen sulfide is the same shape as water (H2O); bent, with bond angles that might be estimated as approx 104.5° (although they are lower). The electron domains form a tetrahedral shape, but two bonds are missing (they are lone pairs).
The Lewis diagram and shape are drawn below. Remember that molecules are three-dimensional, even though we draw the Lewis diagrams in two dimensions on the page.
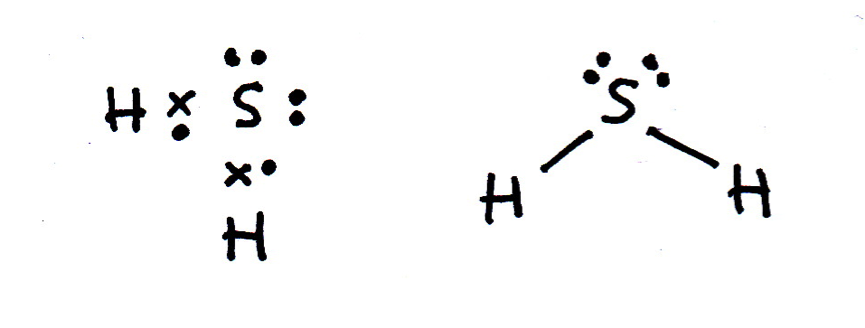
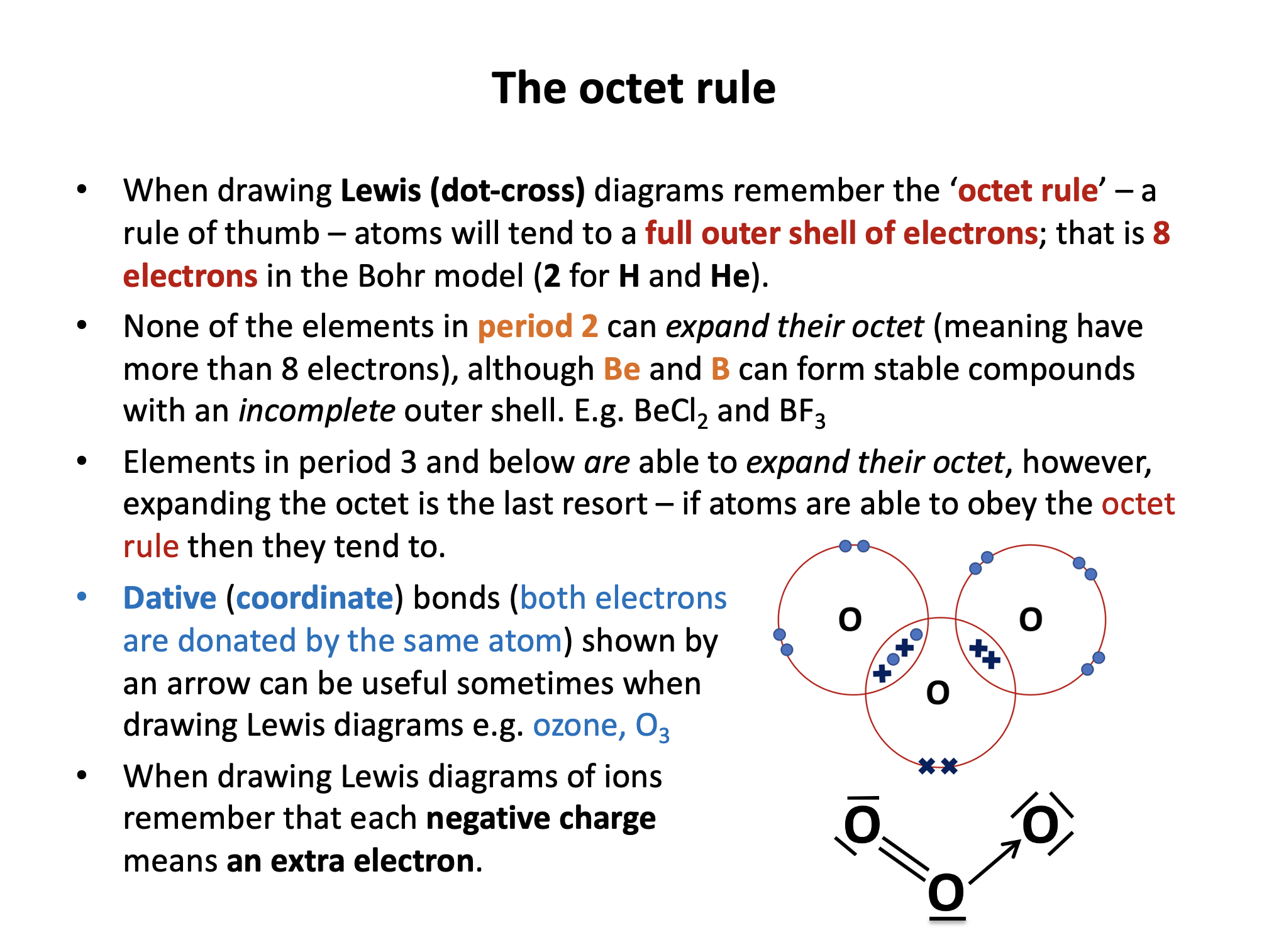
How many electrons in total in the valence (outer) electron shells of boron and beryllium respectively in BCl3 and BeCl2?
Boron and beryllium can form stable molecules with incomplete valence shells. Boron in BCl3 forms only three single bonds with each chlorine; a total of 6 electrons in the valence shell. Beryllium in BeCl2 forms only two single bonds with each chlorine; a total of 4 electrons in the valence shell.
What are the shapes and bond angles around the central boron and beryllium atoms in BCl3 and BeCl2 respectively?
Boron and beryllium can form stable molecules with incomplete valence shells. Boron in BCl3 forms only three single bonds with each chlorine; a total of 6 electrons in the valence shell. Beryllium in BeCl2 forms only two single bonds with each chlorine; a total of 4 electrons in the valence shell.
Thus in BCl3 and BeCl2 respectively the shapes and bond angles are trigonal planar, 120° and linear, 180°.
Look at the table below:
Chemical | Melting/boiling point | Electrical conductivity (solid) | Electrical conductivity (molten) |
| W | high | high | high |
| X | high | nil | high |
| Y | high | nil | nil |
| Z | low | nil | nil |
Which of the four chemicals is likely to be a chemical with a giant covalent structure, like silicon dioxide?
Giant covalent structures are likely to have high melting and boiling points; it takes a lot of energy to break the network of strong covalent bonds. They are also unlikely to conduct electricity in any state (graphite is an exception) as they do not usually have mobile electrons or mobile ions to carry charge.
So Y is the correct answer.
Look at the table below:
Chemical | Melting/boiling point | Electrical conductivity (solid) | Electrical conductivity (molten) |
| W | high | high | high |
| X | high | nil | high |
| Y | high | nil | nil |
| Z | low | nil | nil |
Which of the four chemicals is likely to be a chemical with a simple (molecular) covalent structure?
Simple covalent structures are likely to have low melting and boiling points; it does not take much energy to break the relatively weak intermolecular forces between molecules. They are also unlikely to conduct electricity in any state as they do not usually have mobile electrons or mobile ions to carry charge.
So Z is the correct answer.
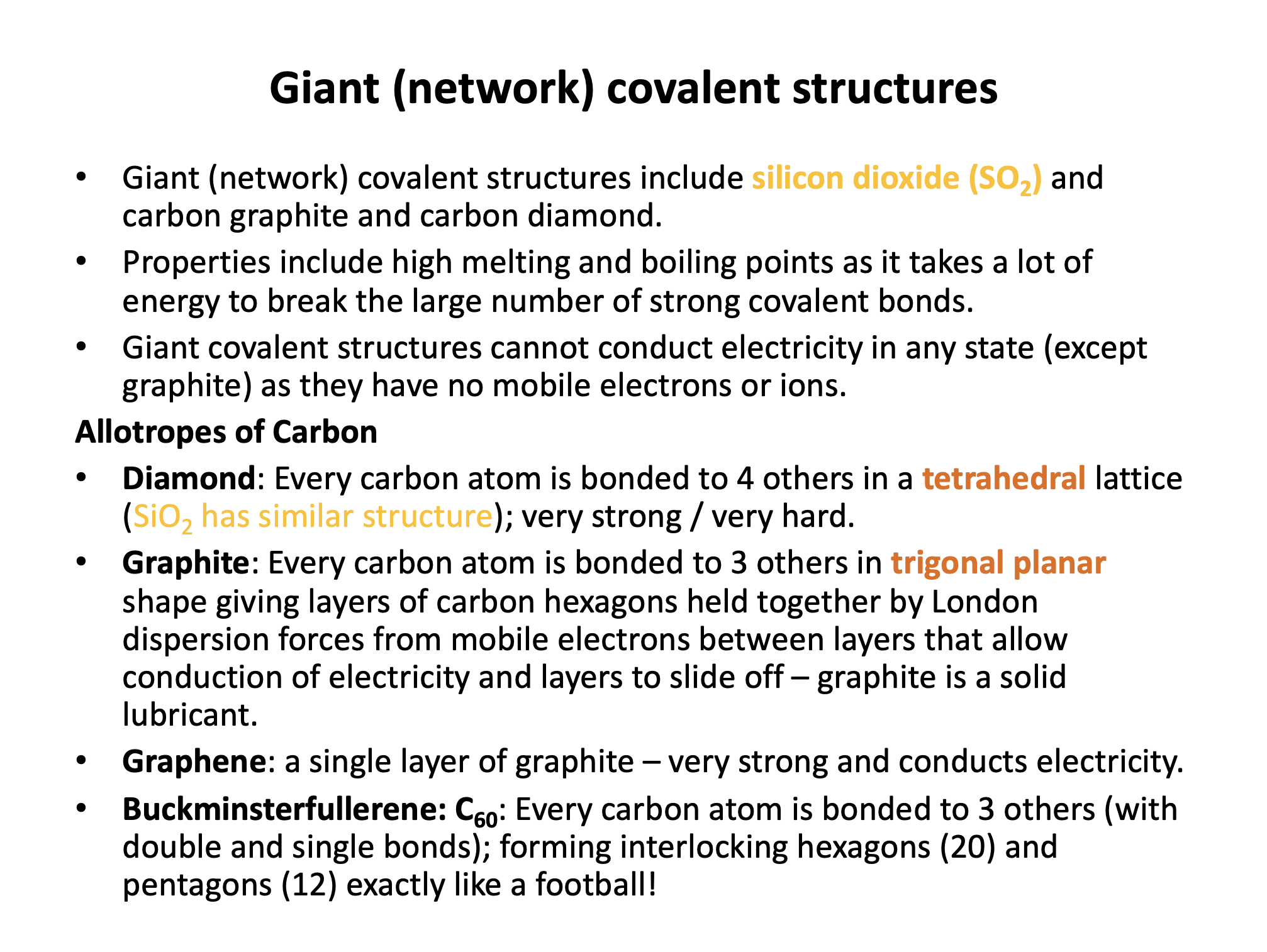
Allotropes of carbon include diamond, graphite and buckminsterfullerene (C60). How many bonds does each carbon form connecting to another carbon atom in each of these allotropes respectively?
In diamond the carbon atoms are bonded in a tetrahedral arrangement to 4 other carbon atoms, in a giant covalent structure.
In graphite the carbon atoms are bonded in a trigonal planar arrangement to 3 other carbon atoms, in a giant covalent structure, with carbon hexagon rings, in layers. (The fourth electron from every carbon atom is delocalised between the layers and holds the layers together.)
In buckminsterfullerene the carbon atoms are bonded in a trigonal planar arrangement to 3 other carbon atoms, forming the surface of the C60 'carbon football' molecule with interlinking carbon hexagons (20) and pentagons (12).
Which is the best description of why graphite in the solid state can conduct electricity and why graphite is a good solid state lubricant respectively?
In graphite the carbon atoms are bonded in a trigonal planar arrangement to 3 other carbon atoms, in a giant covalent structure, with carbon hexagon rings, in layers. The fourth electron from every carbon atom is delocalised between the layers and holds the layers together (weakly).
Graphite conducts electricity in the solid state because the delocalised electrons between layers are able to move and carry charge.
Graphite is a good solid state lubricant because the layers of atoms are able to slide over one-another; the London dispersion forces between the layers are weak and easily broken. Individual atoms cannot move independently, but the layers can easily slide off the structure (think of a pencil marking a piece of paper).
The correct answer is therefore: 'The delocalised electrons are free to move between layers: The layers of atoms in the lattice can slide over one-another'.
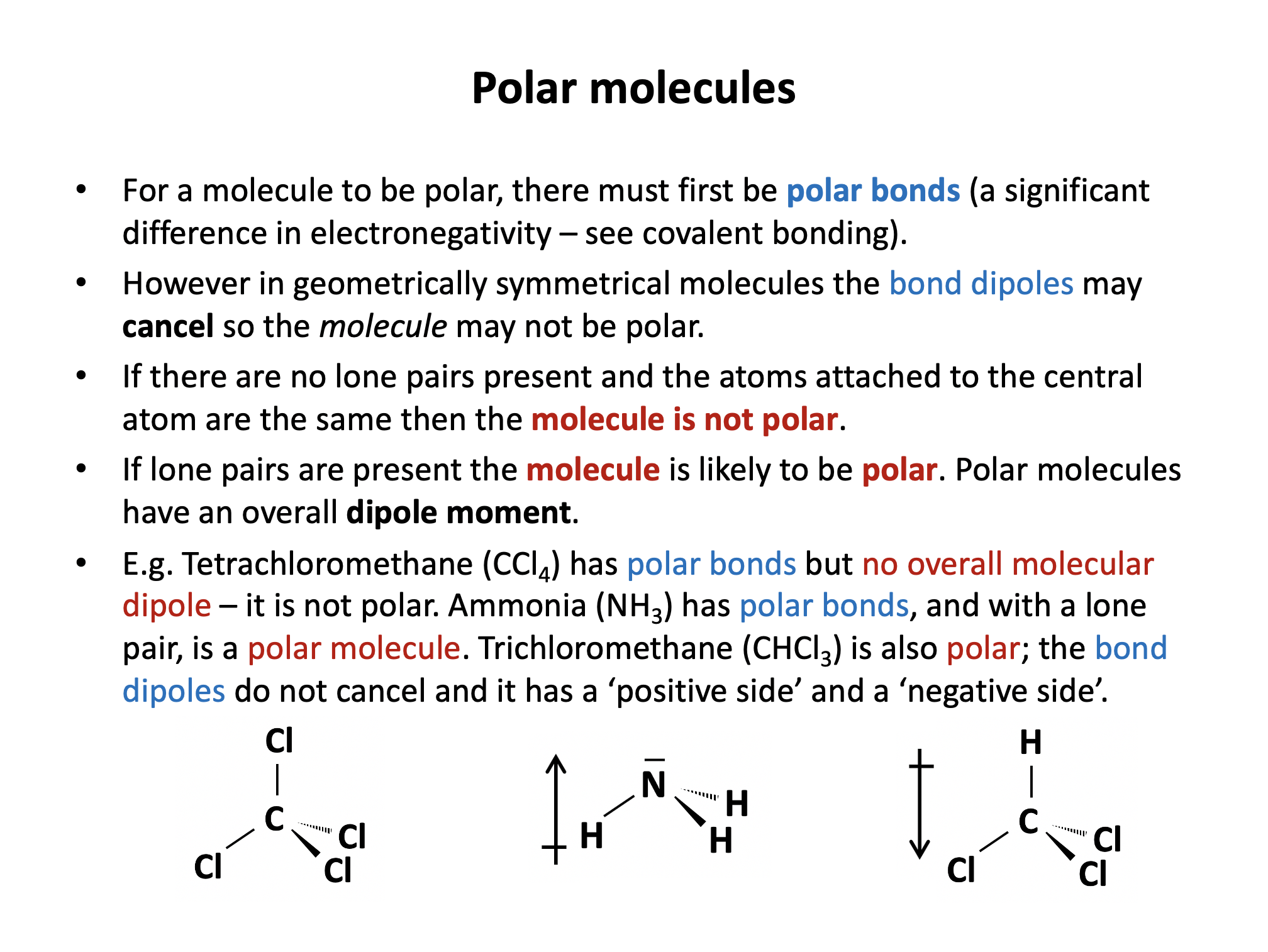
Which of the following molecules is likely to be polar?
To be polar, a molecule must have polar bonds (differences in electronegativity) within it and must have asymmetric geometry (molecules will then have an overall dipole moment). If the dipoles of polar bonds cancel out because the molecule is symmetrical, then the molecule will have no overall dipole.
The C–H bonds are not considered to be significantly polar, and in addition, CH4 and C2H6 both have symmetrical geometry.
The Cδ+–Clδ– bond is polar. In CCl4 the dipoles cancel as the molecule is symmetrical, but in CH3Cl the dipoles will not cancel out and CH3Cl is therefore polar.
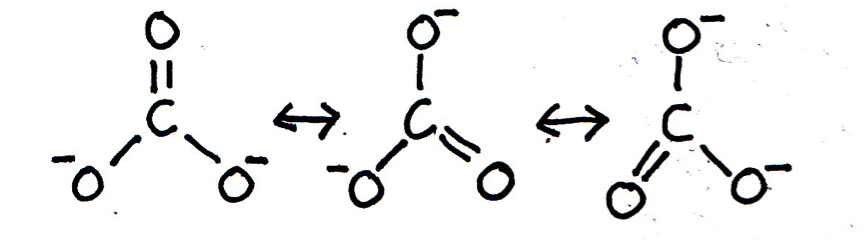
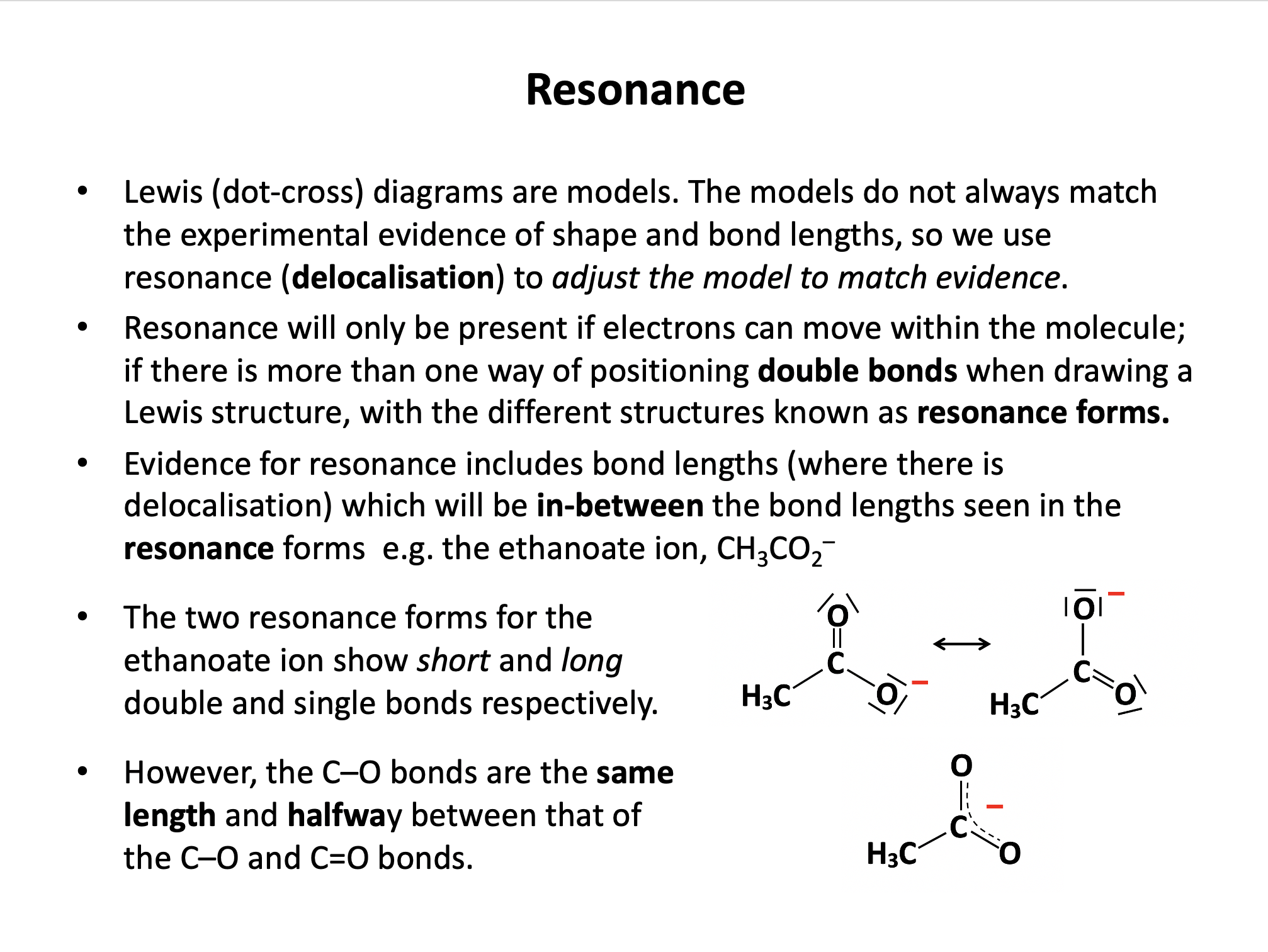
When the bonding in a chemical species cannot be adequately represented by one single Lewis diagram, resonance structures may be used. Which of the following chemical species exhibit resonance?
Lewis (dot-cross) diagrams for C2H6, OH– and CO2 may all be drawn that accurately represent these species in terms of bond length and shape etc. The carbonate ion CO32– is said to exhibit resonance, because the shape of the compound ion with three C–O bonds of equal length cannot be represented by a conventional Lewis diagram. The resonance structures are shown below (showing bonds only for clarity; lone pairs are not shown):
When the bonding in a chemical species cannot be adequately represented by one single Lewis diagram, resonance structures may be used. When the resonance structures for a molecule of ozone, O3, are drawn, how many bonds do each of the three oxygen atoms form?
The ozone molecule is said to exhibit resonance, because the shape of the molecule with two O–O bonds of equal length cannot be represented by a conventional Lewis diagram. The resonance structures are shown below (showing bonds only for clarity; lone pairs are not shown). The oxygen atoms form 2: 3: 1 bonds (or vica versa) respectively. The arrow represents a coordinate (dative) bond.

Paper 1
Core (SL&HL): Bonding and Structure core (SL and HL) paper 1 questions
AHL (HL only): Bonding and Structure AHL (HL only) paper 1 questions
Paper 2
Core (SL&HL): Bonding & Structure core (SL & HL) paper 2 questions
AHL (HL only): Bonding & Structure AHL (HL only) paper 2 questions
How much of Covalent structure have you understood?









 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn