Oxidation & Reduction
- Oxidation and reduction are commonly known as redox reactions
- These reactions occur at the same time and involve the transfer of electrons between molecules
- Oxidation is the loss of electrons
- Reduction is the gain of electrons
- Redox reactions also involve hydrogen, oxygen and energy transfer
- Oxidation is also the loss of hydrogen, gain of oxygen and releases energy to the surroundings (exergonic)
- Reduction is also the gain of hydrogen, loss of oxygen and absorbs energy from the surroundings (endergonic)
- Molecules that have a strong tendency to lose/donate their electrons, are known as reducing agents
- Molecules that that have a strong tendency to gain electrons, are known as oxidising agents
- Oxidation and reduction reactions feature in cellular respiration and photosynthesis
Table comparing oxidation and reduction
Oxidation and reduction in cell respiration
- Respiration involves a group of molecules called electron carriers which accept or donate their electrons
- NAD+ is the primary electron carrier involved in respiration
- FAD is another electron carrier used in respiration
- Both NAD and FAD serve as oxidising agents:
- NAD+ and FAD gain electrons and also gain one or more hydrogen ions (from molecules involved in respiration), switching to a slightly different form called reduced NAD and reduced FAD
- NAD+ + 2e- + 2H+ --> NADH + H+
- FAD + 2e- + 2H+ --> FADH2
- These electron carriers are used to transport the electrons they have gained to other reactions in respiration
- When they lose these electrons they return to their original form releasing their electrons in the process
- NADH --> NAD+ + 2e- + 2H+
- FADH2 --> FAD + 2e- + 2H+
- This is an example of a redox reaction
Exam Tip
To help you remember which way around loss and gain of electrons is from redox reactions, think OILRIG:
- Oxidation Is Loss
- Reduction Is Gain
NAD is a collective term for the different forms NAD takes; NAD exists in an oxidised and a reduced form:
- NAD+ is the oxidised form and acts as an oxidising agent
- NADH is the reduced form and acts a reducing agent
Phosphorylation
- Phosphorylation occurs when a phosphate ion is added to a molecule
- E.g. the phosphorylation of ADP to make ATP
- This makes the molecule less stable and therefore more likely to react
- We can say that phosphorylation activates a molecule because it makes it more reactive
- There are two main types of phosphorylation:
- Substrate level phosphorylation where the phosphate ion is transferred from a donor compound
- This takes place in glycolysis and the Krebs cycle
- Oxidative phosphorylation where phosphorylation is coupled with oxidation
- This takes place in the the electron transport chain
- Substrate level phosphorylation where the phosphate ion is transferred from a donor compound
- Phosphorylation is an endergonic reaction whereas the removal of the phosphate ion by hydrolysis in dephosphorylation is an exergonic reaction
- Remember that the hydrolysis of ATP to ADP releases energy, therefore dephosphorylation of ADP is exergonic
- The addition of a phosphate ion to one molecule occurs at the same time as the removal of a phosphate from another; this is known as the coupling of reactions
- E.g. the exergonic dephosphorylation of ATP is coupled with the endergonic phosphorylation of glucose at the start of glycolysis
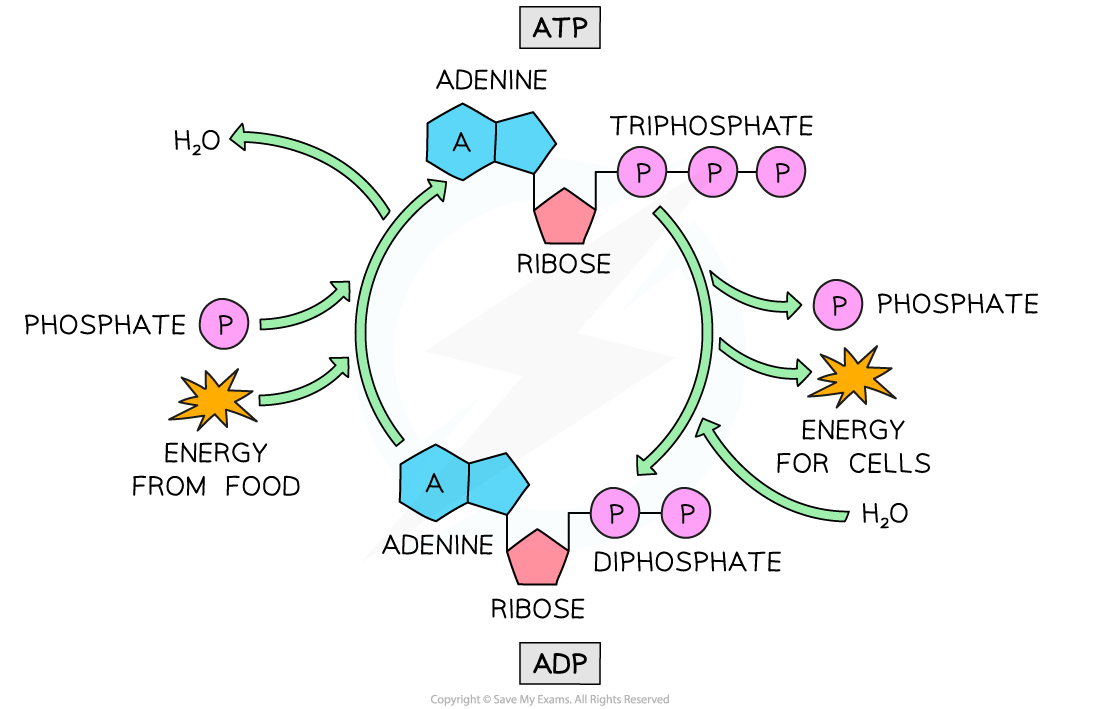
The cyclic formation of ATP from ADP by phosphorylation