Primary Structure
Levels of Protein Structure
- Proteins are relatively large, complex molecules that contain one or more chains of amino acids known as polypeptides
- The three-dimensional arrangement of polypeptide chains dictates a protein's structure and function
- There are four levels of structure in proteins
- Three levels are structural aspects of a single polypeptide chain
- The fourth level relates to a protein that has more than polypeptide chain
Primary structure
- The sequence of amino acids bonded by covalent peptide bonds is the primary structure of a protein
- The DNA of a cell determines the primary structure of a protein by instructing the cell to add certain amino acids in specific quantities in a specific, ordered sequence
- This affects the shape, and therefore the function, of the protein
- The primary structure is specific for each protein
- Some mutations can lead to the incorrect amino acid being incorporated into the polypeptide chain which can affect the function of the protein
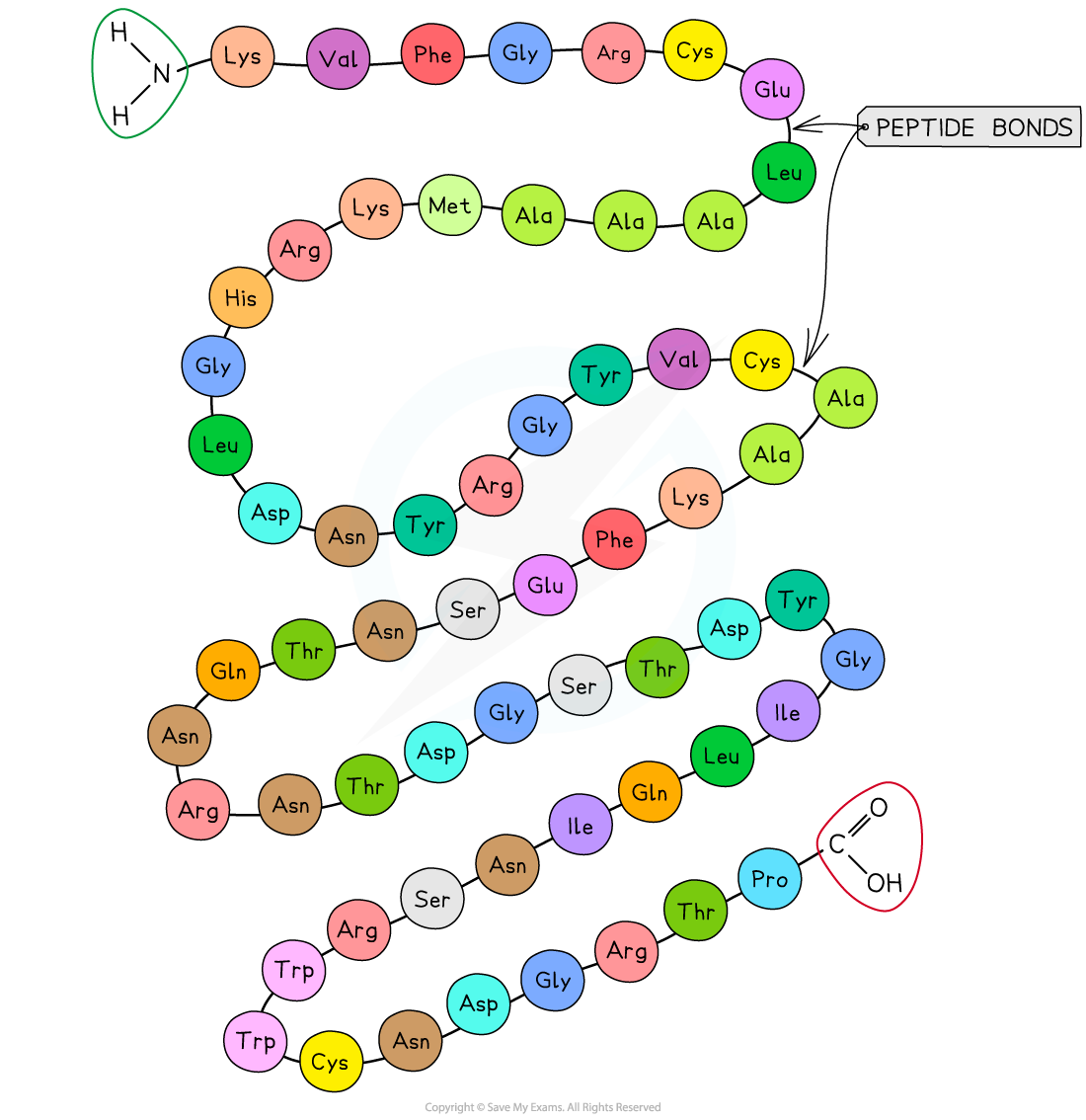
The primary structure of a protein. The three-letter abbreviations indicate the specific amino acid (there are 20 commonly found in cells of living organisms).
Secondary Structure
- Secondary structure is the formation of complex shapes within the polypeptide chain
- Secondary structure of a protein occurs due to weak hydrogen bonds
- Hydrogen bonds form between carboxyl (C=O) groups and amino (H-N-H) groups
- The bonds usually form between non-adjacent amino acids resulting in a change in shape of the linear polypeptide chain
- There are two shapes that can form within proteins due to the hydrogen bonds:
- Alpha-helix (or α-helix)
- Beta-pleated sheet (or β-pleated sheet)
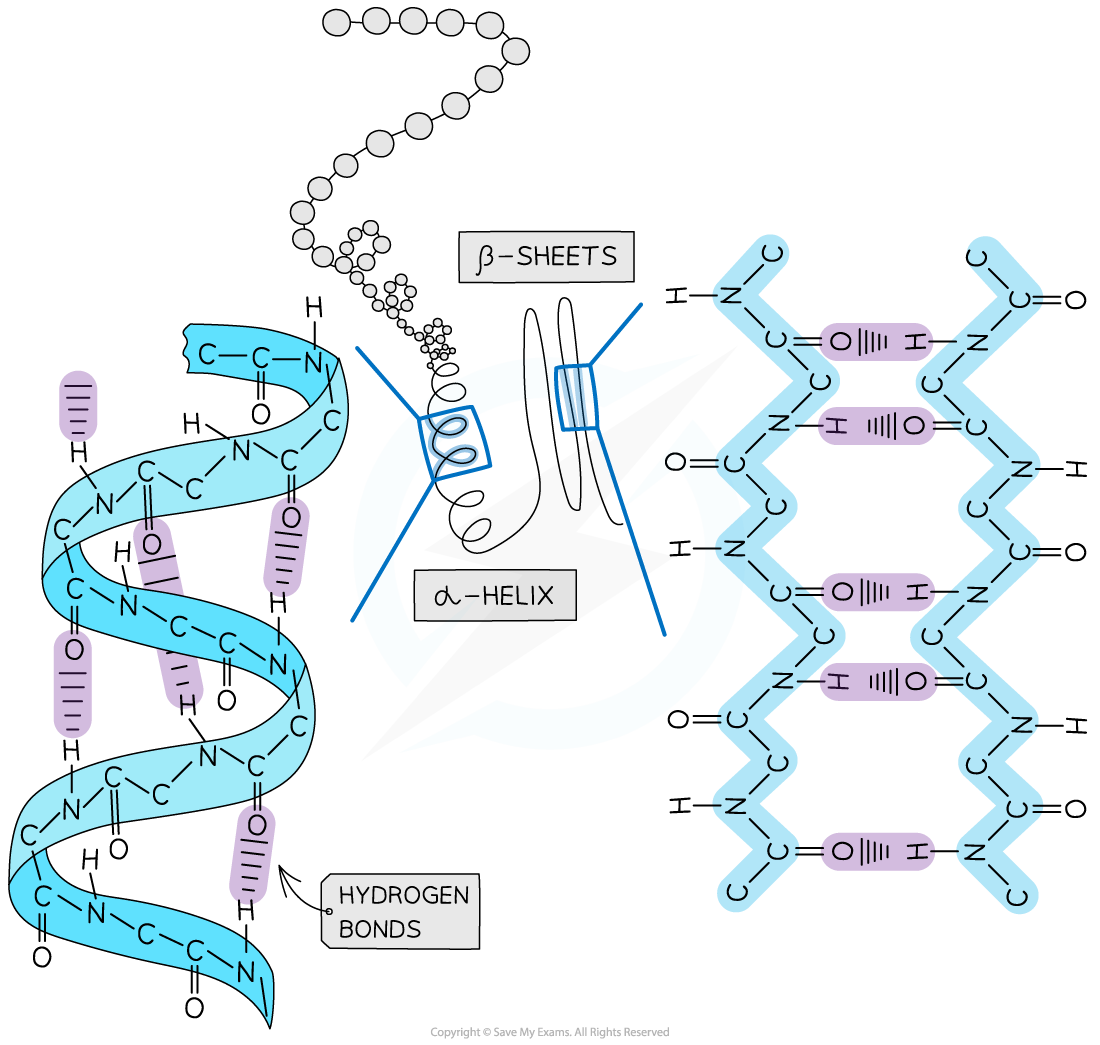
The secondary structure of a protein with the α-helix and β-pleated sheets.
The magnified regions illustrate how the hydrogen bonds form between peptide bonds.
The magnified regions illustrate how the hydrogen bonds form between peptide bonds.
Tertiary Structure
Polar and non-polar amino acids are relevant to the bonds formed between R groups
- Tertiary structure refers to how the polypeptide chain folds to form a complex, three-dimensional shape
- Tertiary structure gives proteins a very specific shape that is important for function
- Such as receptor sites on cell membranes and active sites in enzymes
- Folding results from interactions between R groups (side chains) of the amino acids and the surrounding environment
- A number of different interactions between R-groups contribute to the tertiary structure
- Hydrogen bonds form between polar R-groups
- Hydrophobic interactions form between the R-groups of non-polar amino acids within the interior of proteins to avoid contact with water
- Covalent bonds form between the R-groups of cysteine amino acids to form disulphide bridges
- Ionic bonds form between positively and negatively charged R-groups
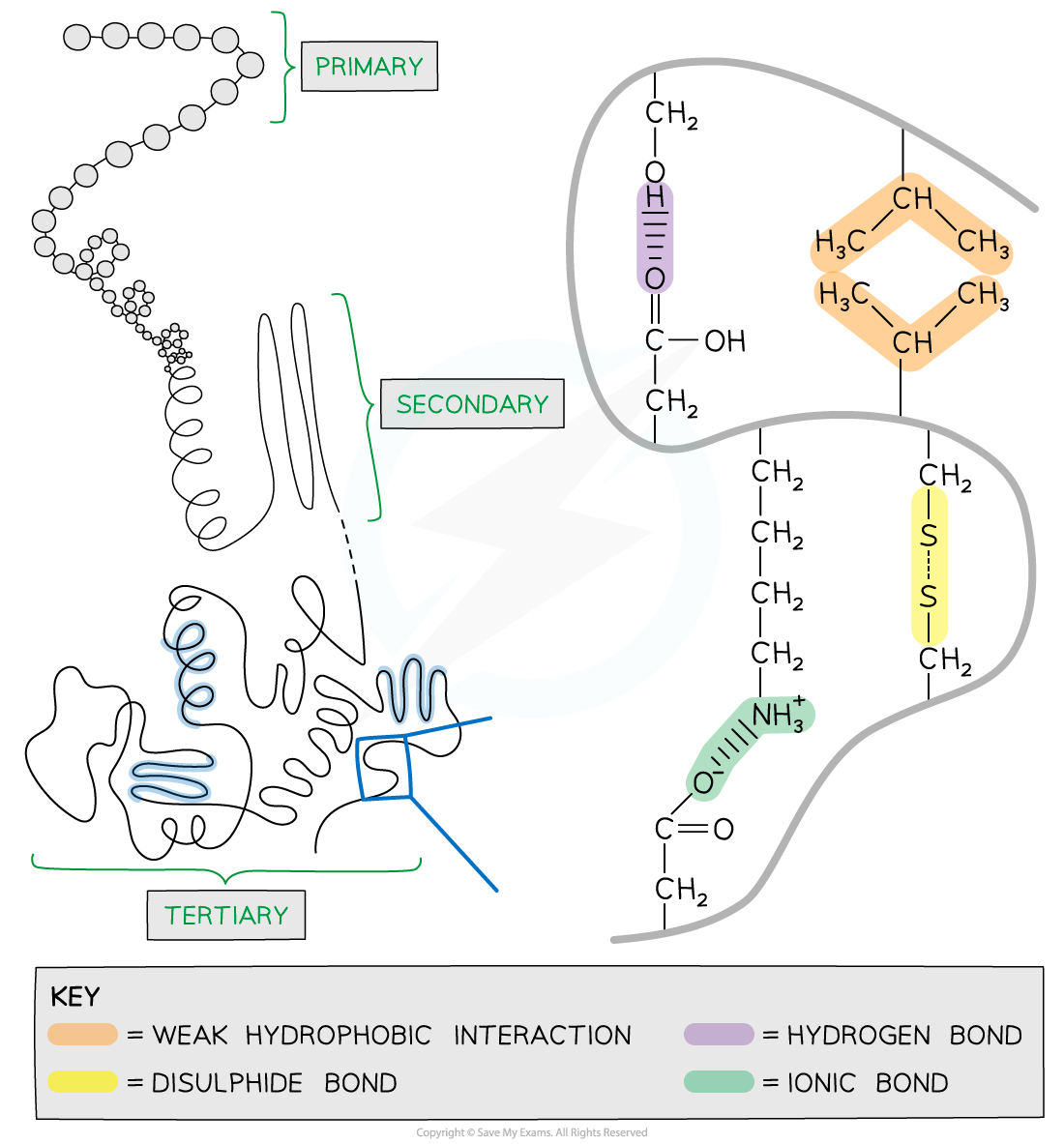
The interactions that occur between the R groups of amino acids determine the tertiary structure and function of a protein
Quaternary Structure
Quaternary structure
- Large proteins often consist of multiple polypeptide chains functioning together as a larger biologically active macromolecule
- Each polypeptide chain is referred to as a subunit of the protein
- Many proteins also contain non-polypeptide components (prosthetic groups) and are classed as conjugated proteins
- Quaternary structure refers to how polypeptides and other components are arranged
- This relates closely to function
- Proteins with only one polypeptide chain do not have a quaternary structure
- Haemoglobin is a conjugated protein, having quaternary structure, as it consists of multiple polypeptide chains (making four subunits) each with a prosthetic group
- There are two pairs of identical polypeptide chains (α–globins and β–globins)
- Each subunit has a prosthetic haem group which contains an iron atom (Fe)
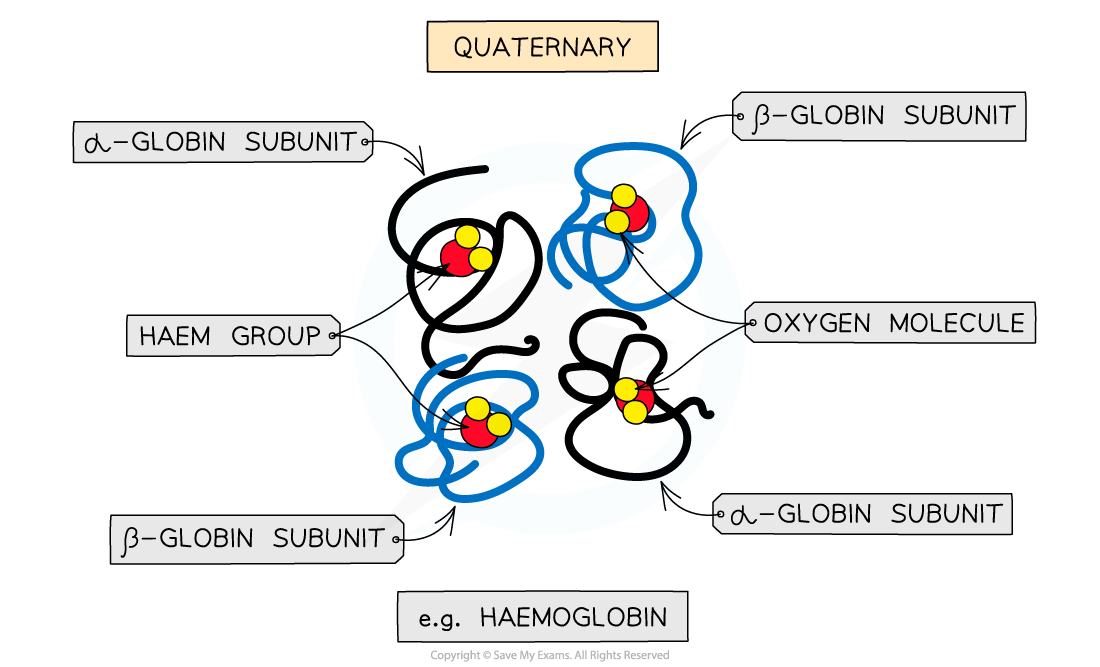
The quaternary structure of haemoglobin
Four subunits (polypeptide chains) and prosthetic haem groups work together to carry oxygen
Four subunits (polypeptide chains) and prosthetic haem groups work together to carry oxygen
Summary of Bonds in Proteins Table
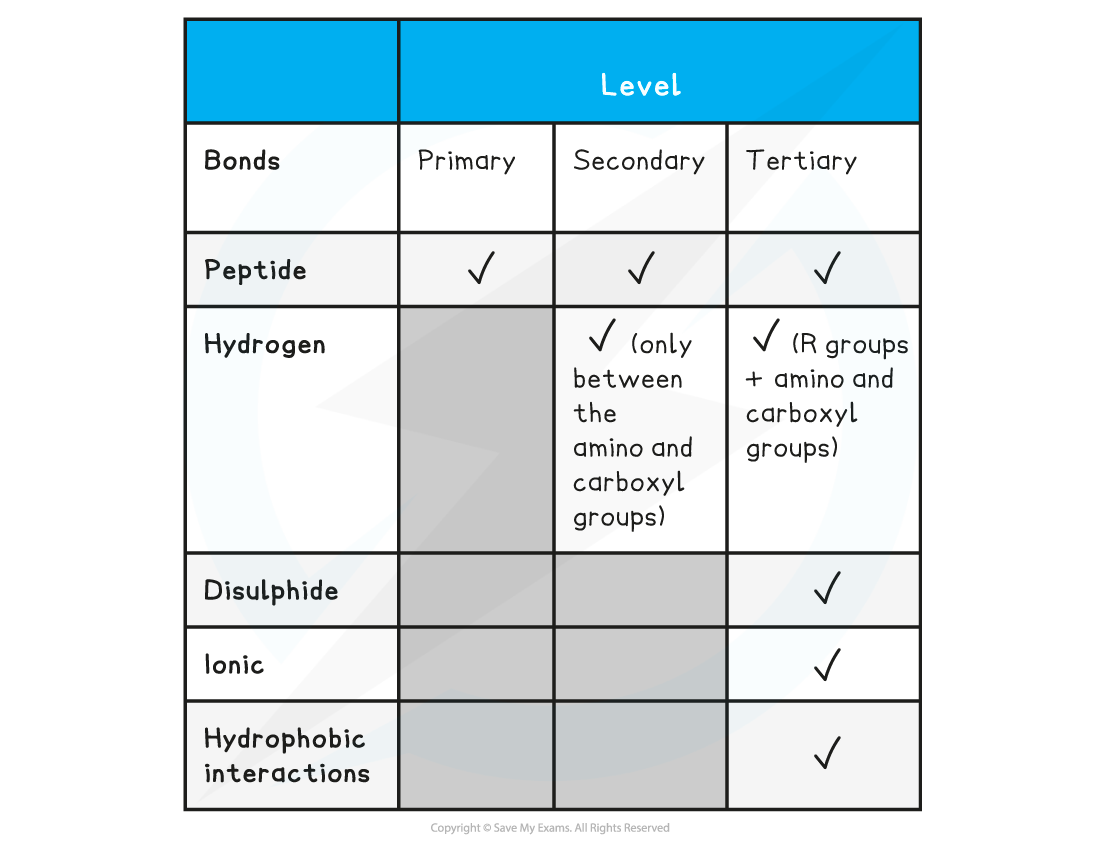
Exam Tip
Familiarise yourself with the difference between the four structural levels found in proteins, noting which bonds are found at which level. Remember that the hydrogen bonds in tertiary structures are between the R groups whereas in secondary structures the hydrogen bonds form between the amino and carboxyl groups.
