Hydrogen Bonds
- Hydrogen bonding plays an important role between many biological molecules
- Some key functions include:
- Dissolving of solutes in water
- The cohesion and adhesion of water molecules
- These properties allow water to move up the trunks of really tall trees
- Base-pairing between the two strands of DNA
- Structure:
- Hydrogen bonds help to form part of the secondary and tertiary levels of structure in proteins
- The hydrogen bonds found between strands of cellulose and collagen give those molecules their tensile strength
- Interactions between mRNA and tRNA during protein synthesis
- Surface effects on membranes between polar phosphate groups and water
Hydrogen bonding in water
- Hydrogen bonding is a fundamental property of water
- Water is of the utmost biological importance
- It is the medium in which all metabolic reactions take place in cells
- Between 70% to 95% of the mass of a cell is water
- Water is so fundamental to life that astronomers look for signs of water on other planets and moons, as indicators of possible extra-terrestrial life
- As 71% of the Earth’s surface is covered in water it is a major habitat for organisms
- Water is composed of atoms of hydrogen and oxygen
- One atom of oxygen combines with two atoms of hydrogen by sharing electrons (covalent bonding)
- Although water as a whole is electrically neutral, the sharing of the electrons is uneven between the oxygen and hydrogen atoms
- The oxygen atom attracts the electrons more strongly than the hydrogen atoms, resulting in a weak negatively charged region on the oxygen atom (δ-) and a weak positively charged region on the hydrogen atoms(δ+), this also results in the molecule's asymmetrical shape
- This separation of charge due to the electrons in the covalent bonds being unevenly shared is called a dipole
- When a molecule has one end that is negatively charged and one end that is positively charged it is also a polar molecule
- Water is therefore a polar molecule
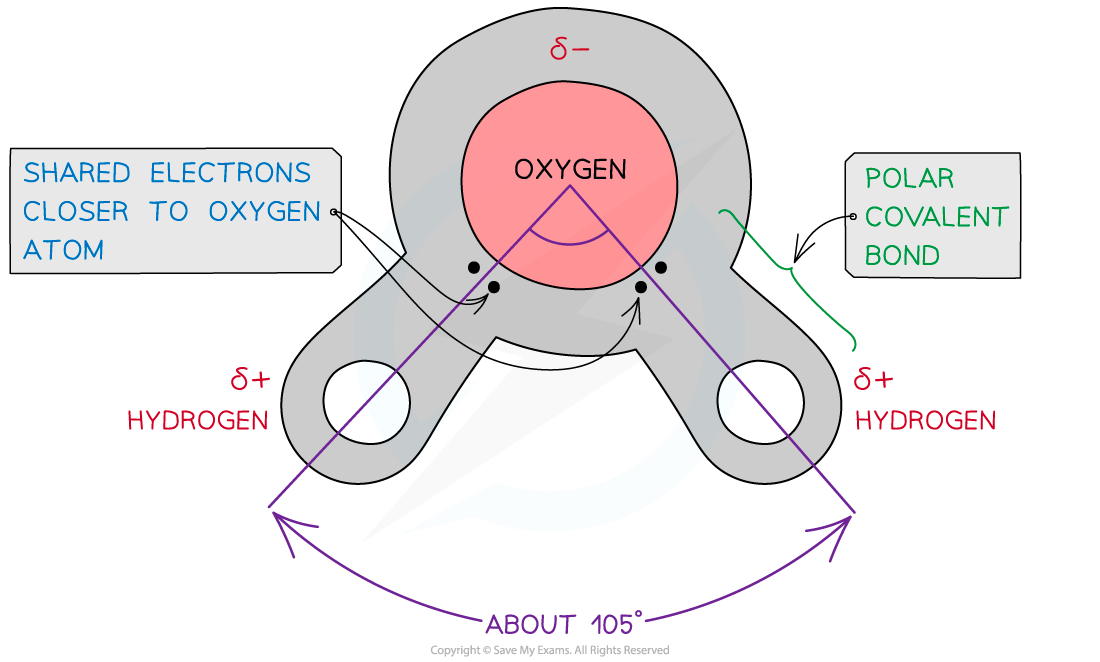
The covalent bonds of water make it a polar molecule
- Hydrogen bonds form between water molecules
- As a result of the polarity of water, hydrogen bonds form between the positive and negatively charged regions of adjacent water molecules
- Hydrogen bonds are weak, when there are few, so they are constantly breaking and reforming
- However, when there are large numbers present they form a strong structure
- Hydrogen bonds cause many of the properties of water molecules, that make them so important to living organisms:
- Excellent solvent – many polar substances can dissolve in water
- A relatively high specific heat capacity
- A relatively high latent heat of vaporisation
- Water is less dense when a solid (ice floats, allowing aquatic life to flourish beneath)
- Water has high surface tension and cohesion
- It acts as a reagent
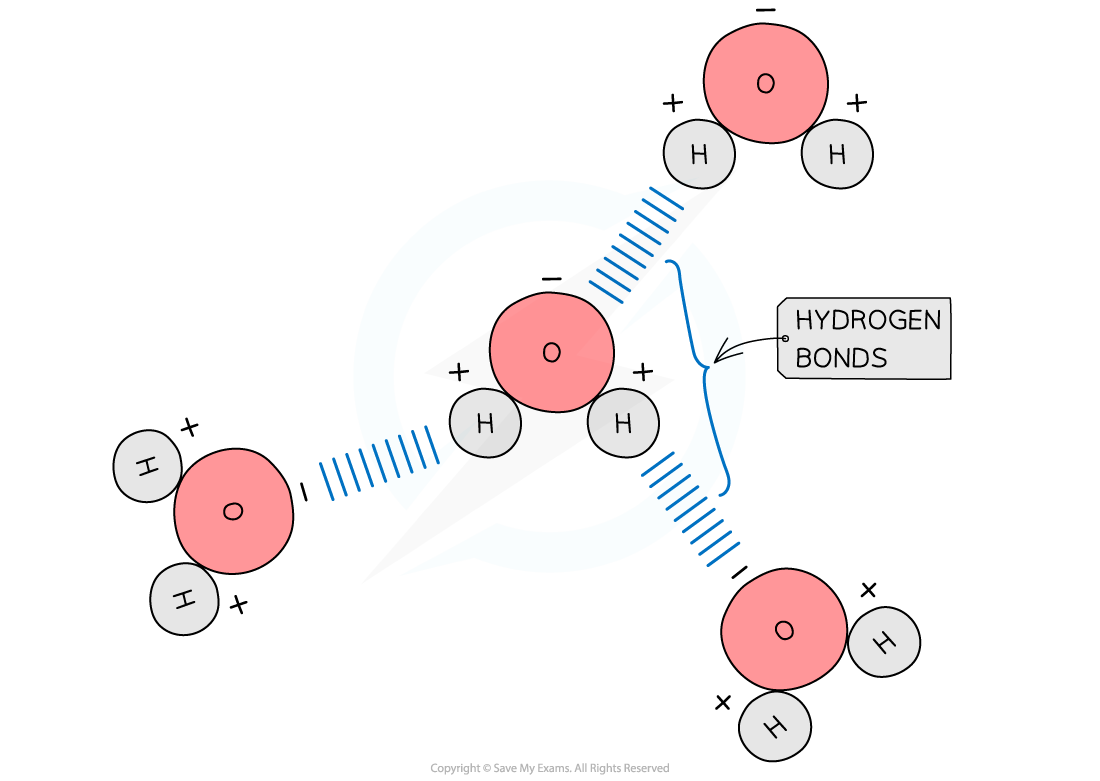
The polarity of water molecules allows hydrogen bonds to form between adjacent water molecules
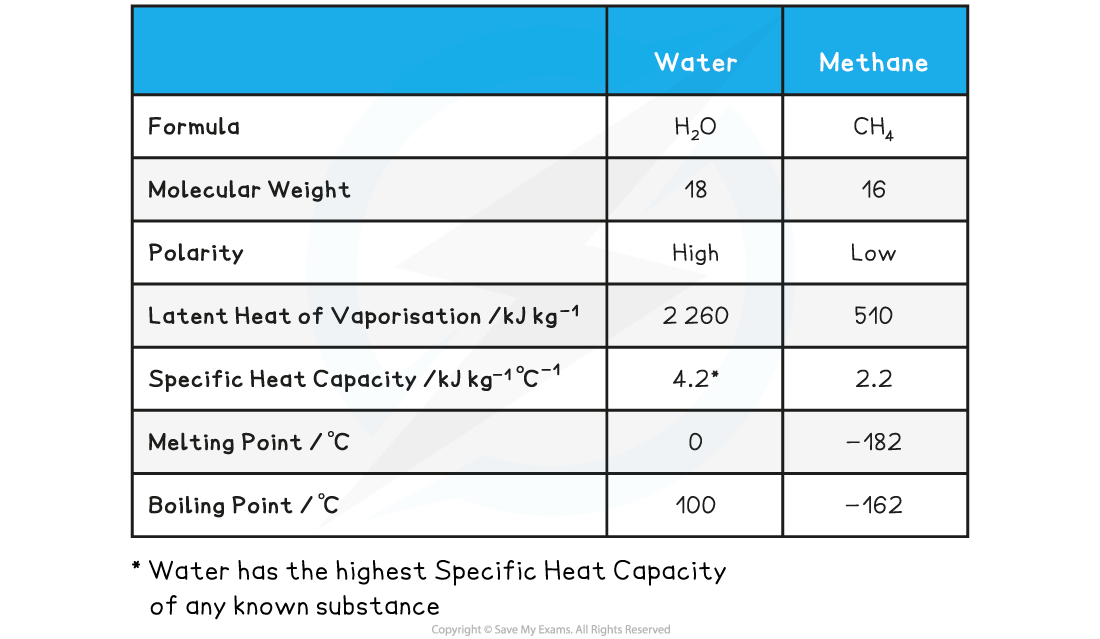
Comparison: Water & Methane
- Both methane (CH4) and water (H2O) are small, covalently-bonded molecules
- Methane is the simplest organic compound
- They have similar molecular weights, 16 and 18 respectively
- But water is polar and so forms hydrogen bonds, whereas methane is not polar and so does not form hydrogen bonds
- Water is liquid at room temperature, whereas methane is a gas
- Other compounds, with a small molecular weight, such as ammonia (NH3) and carbon dioxide (CO2) are gases at room temperature
- These compounds also do not form hydrogen bonds
- This shows how water molecules are held together by hydrogen bonds, whereas the other gas molecules are free to move around in the gaseous state
- Methane is a fuel with a high energy content in its bonds, whereas water is a final product of combustion and respiration
- Other compounds, with a small molecular weight, such as ammonia (NH3) and carbon dioxide (CO2) are gases at room temperature
- Understanding the molecular properties of methane underlines the significance of hydrogen bonding in water
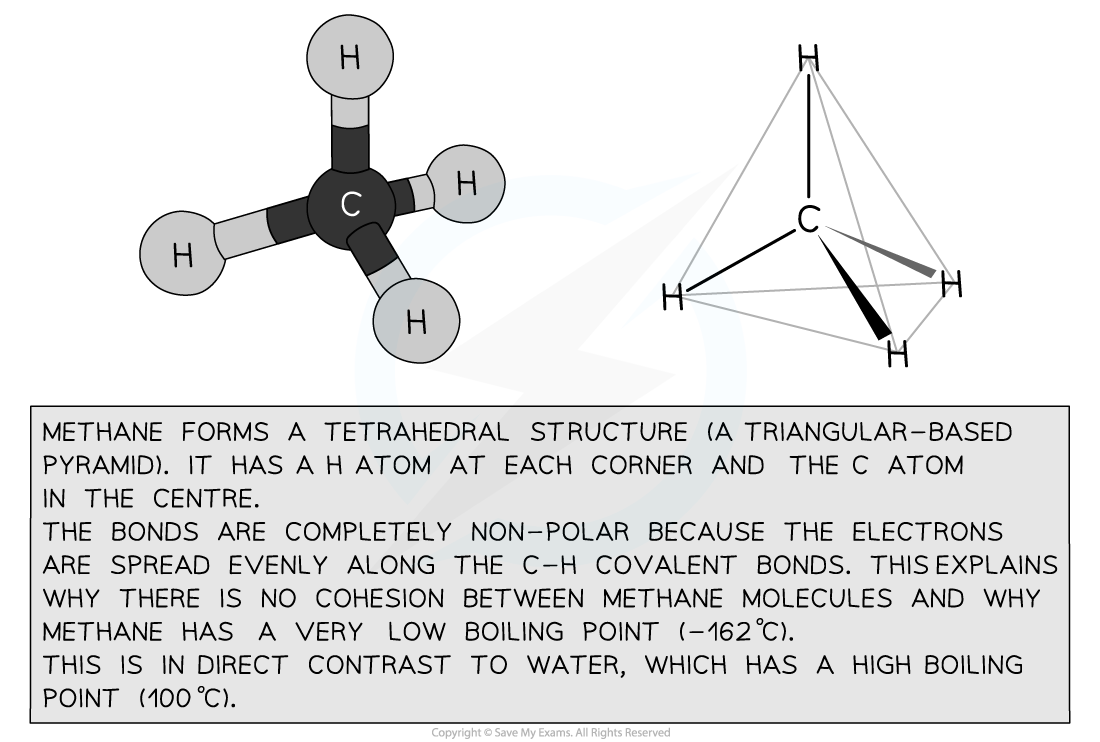
Methane’s structure determines its properties
Comparison of the Properties of Water and Methane Table
Exam Tip
It is important to know where the hydrogen bonds form between water molecules (oxygen of one water molecule to the hydrogen atom of another).
