Formation of Sugars
- Monosaccharides can join together via condensation reactions to form disaccharides
- A condensation reaction is one in which two molecules join together via the formation of a new chemical bond, with a molecule of water being released in the process
- The new chemical bond that forms between two monosaccharides is known as a glycosidic bond
- To calculate the chemical formula of a disaccharide, you add all the carbons, hydrogens and oxygens in both monomers then subtract 2 H and 1 O (for the water molecule lost)
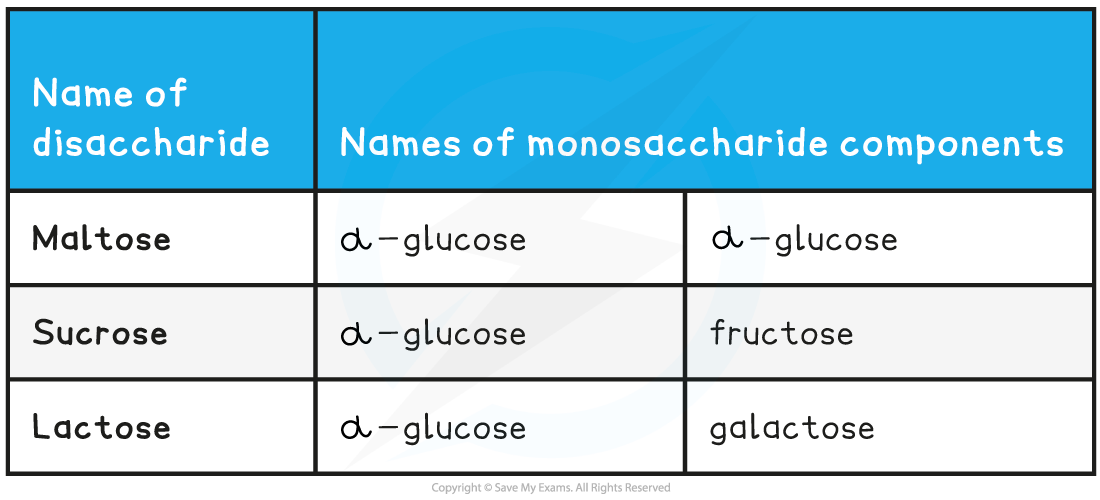
- Common examples of disaccharides include:
- Maltose (the sugar formed in the production and breakdown of starch)
- Sucrose (the main sugar produced in plants)
- Lactose (a sugar found only in milk)
- All three of the common examples above have the formula C12H22O11
Common Disaccharides and their Monosaccharide Monomers Table
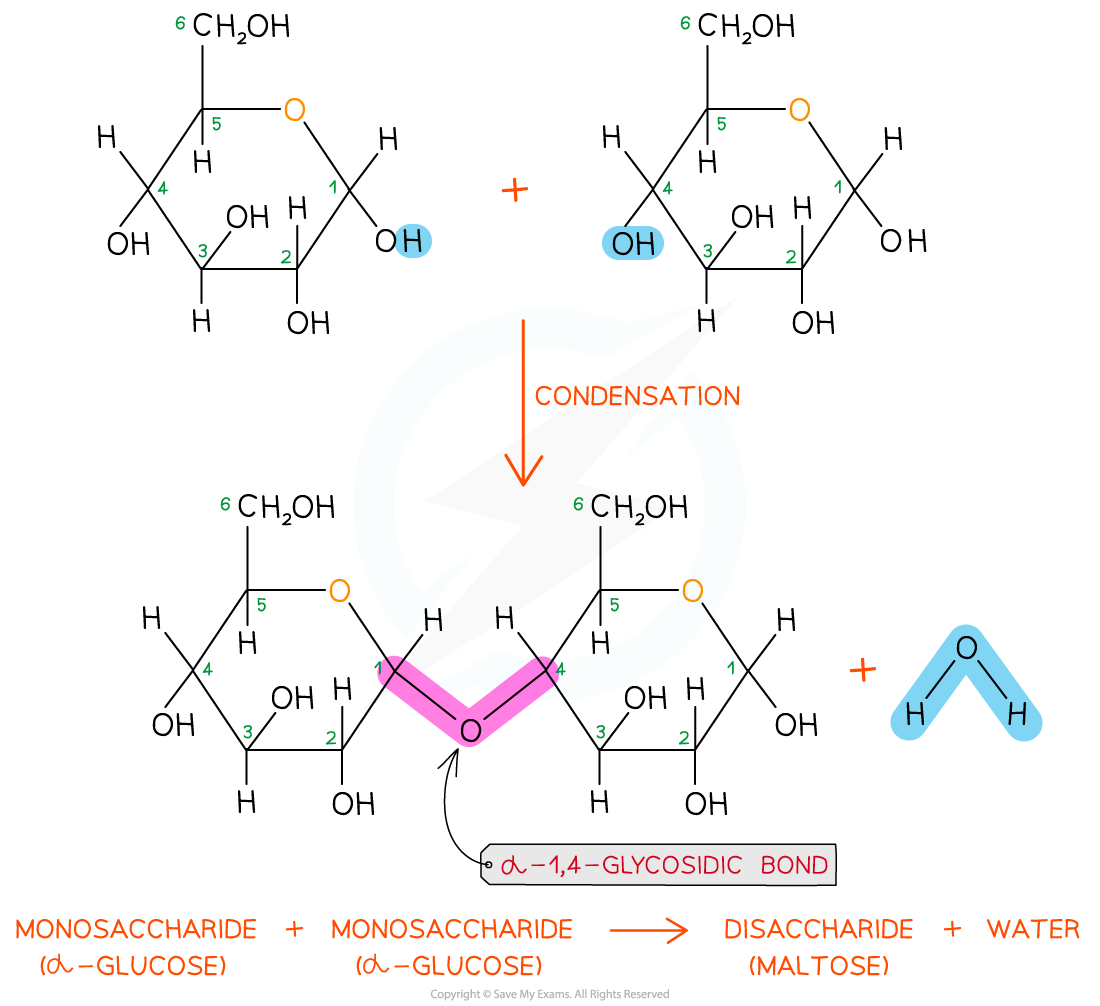
The disaccharide maltose is formed from two α-glucose monomers (sub-units)
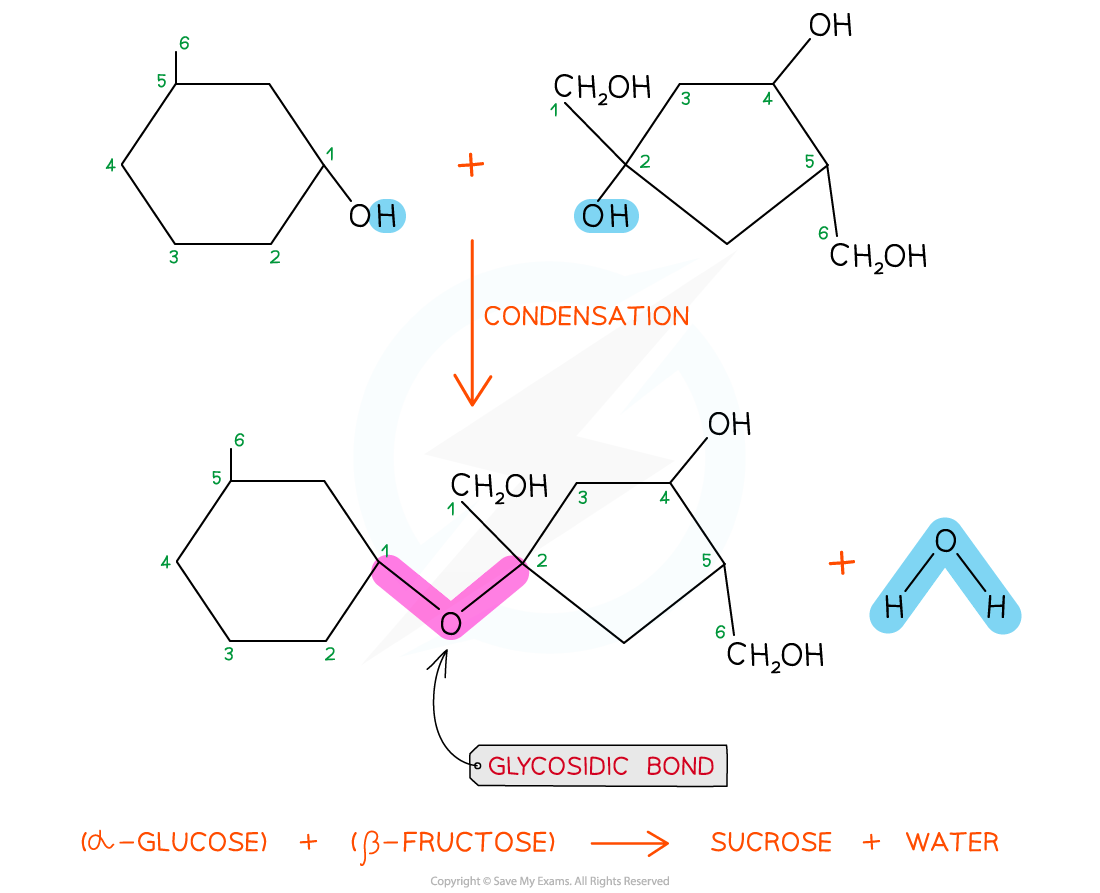
The disaccharide sucrose is formed from α-glucose and fructose monomers (sub-units)
Exam Tip
Galactose and fructose are monosaccharides and actually have the same molecular formula as glucose. However, the atoms that make up these three monosaccharides are arranged in different ways, meaning they each have slightly different molecular structures, giving them slightly different properties. For example, fructose is sweeter in taste than glucose. The three sugars are isomers.
Polysaccharides: Structure & Function
- Starch, cellulose and glycogen are polysaccharides
- Polysaccharides are macromolecules that are polymers formed by many monosaccharides joined together by glycosidic bonds
- The bonds form from condensation reactions, resulting in polysaccharide chains
- These chains may be:
- Branched or unbranched
- Folded (making the molecule compact which is ideal for storage eg. starch and glycogen)
- Straight (making the molecules suitable to construct cellular structures e.g. cellulose) or coiled
- Starch and glycogen are storage polysaccharides because they are:
- Compact (so large quantities can be stored)
- Insoluble (so will have no osmotic effect)
- The monosaccharide glucose lowers the osmolarity of a cell causing water to move into cells
- If too much water enters an animal cell it will burst
- Plant cells have developed thicker cell walls to prevent this
- Cellulose is a structural polysaccharide because it is:
- Strong and durable
- Insoluble and slightly elastic
- Chemically inert (hardly any organisms possess enzymes that can hydrolyse it)
- Is an ideal material for plant cell walls
- The main constituent of dietary fibre for animals that eat plants
Starch
- Starch is the storage polysaccharide of plants
- It is stored as granules in plastids (e.g. chloroplasts)
- Due to the many monomers in a starch molecule, it takes longer to digest than glucose
- Starch is constructed from two different polysaccharides:
- Amylose (10 - 30% of starch)
- Unbranched helix-shaped chain with 1,4 glycosidic bonds between α-glucose molecules
- The helix shape enables it to be more compact and thus it is more resistant to digestion
- Amylose (10 - 30% of starch)
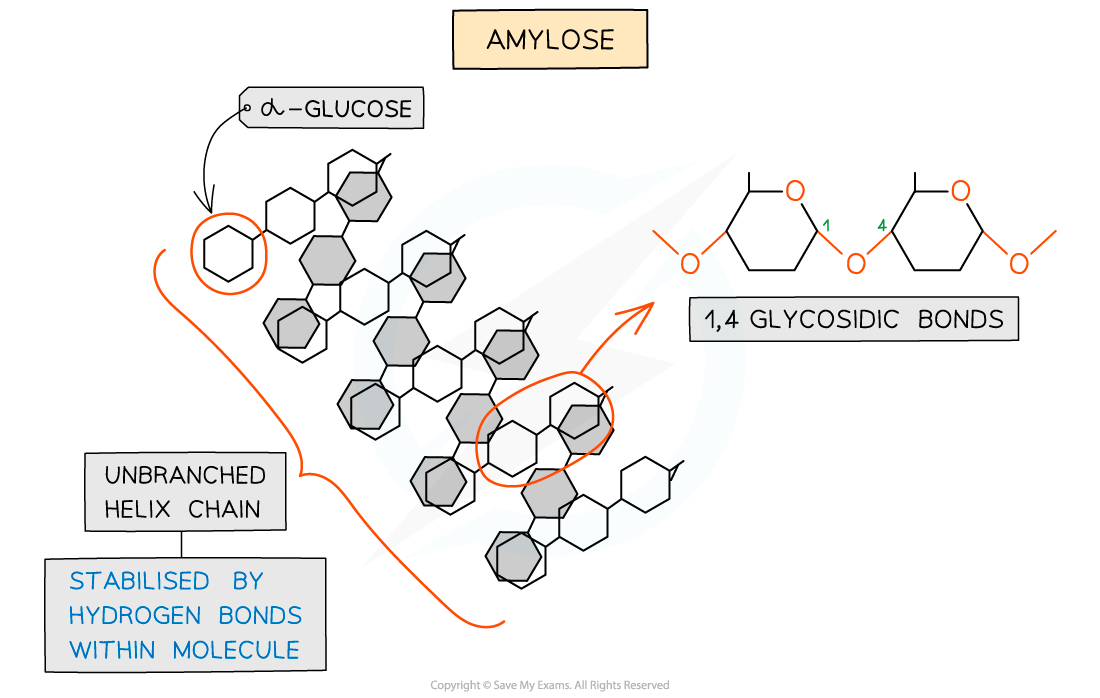
Amylose – one of the two polysaccharides that is used to form starch (the storage polysaccharide in plants)
- Amylopectin (70 - 90% of starch)
- 1,4 glycosidic bonds between α-glucose molecules as well as 1,6 glycosidic bonds creating a branched molecule
- The branches result in many terminal glucose molecules that can be easily hydrolysed, for use during cellular respiration or added to for storage
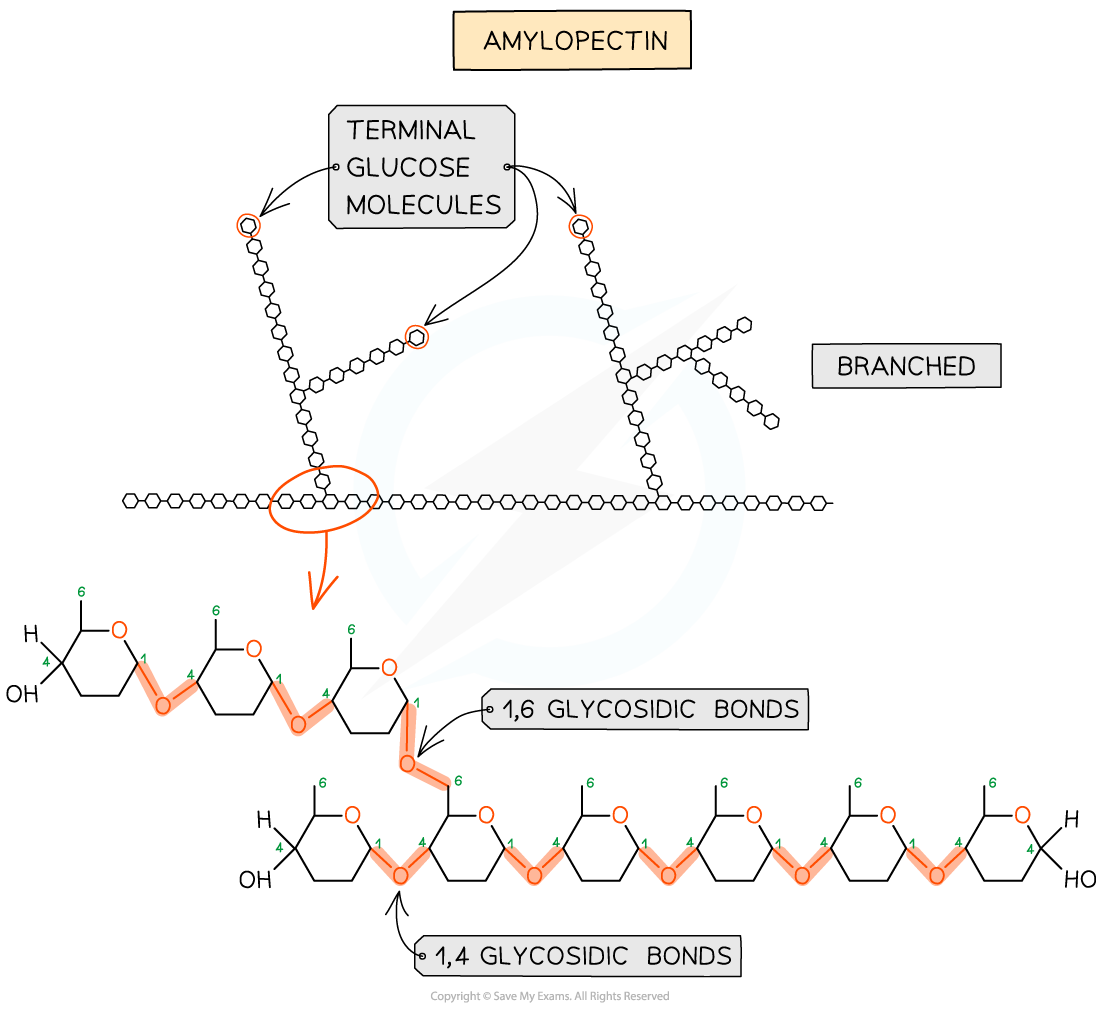
Amylopectin – one of the two polysaccharides that is used to form starch (the storage polysaccharide in plants)
Glycogen
- Glycogen is the storage polysaccharide of animals and fungi, it is highly branched and not coiled
- Liver and muscles cells have a high concentration of glycogen present as visible granules as the cellular respiration rate is high in these cells (due to animals being mobile)
- Glycogen is more branched than amylopectin making it more compact which helps animals store the molecule more efficiently
- The branching enables more free ends where glucose molecules can either be added or removed allowing for condensation and hydrolysis reactions to occur more rapidly – thus the storage or release of glucose can suit the demands of the cell
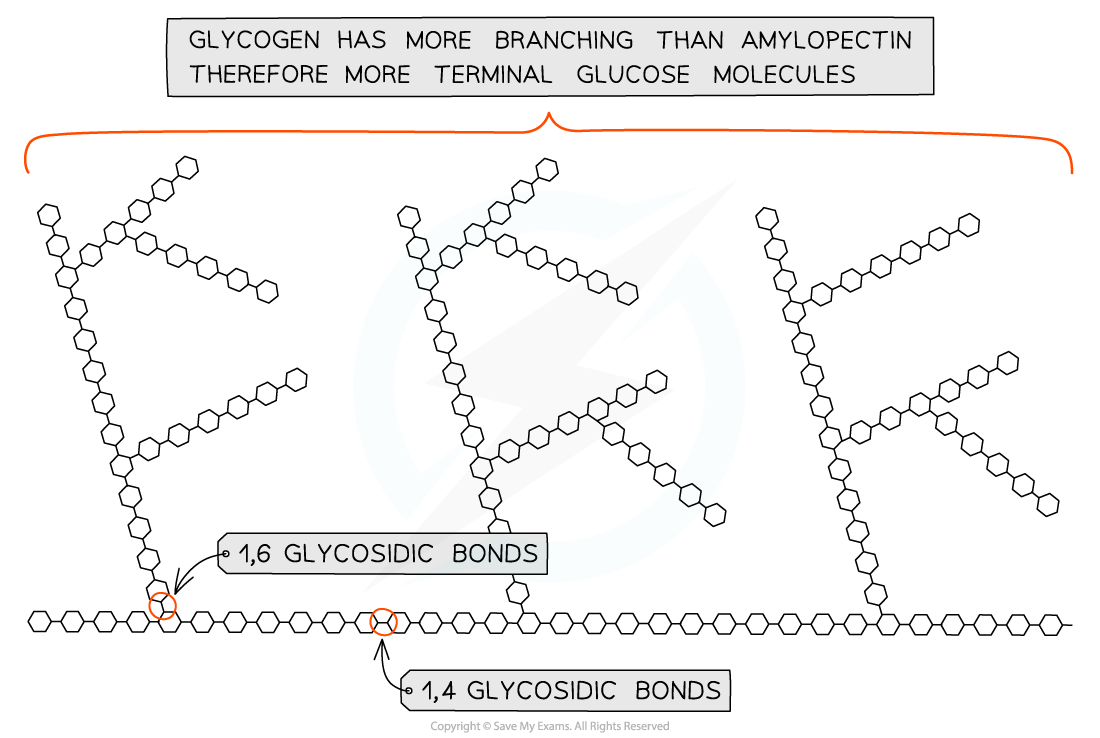
Glycogen, the highly branched molecule used as a storage polysaccharide in animals and fungi
Cellulose
- Cellulose is a polymer of β-glucose monomers
- β-glucose differs very slightly in structure to α-glucose
- The hydroxyl group on the C1 atom sits above the carbon ring in β-glucose, whereas it sits below the ring in α-glucose
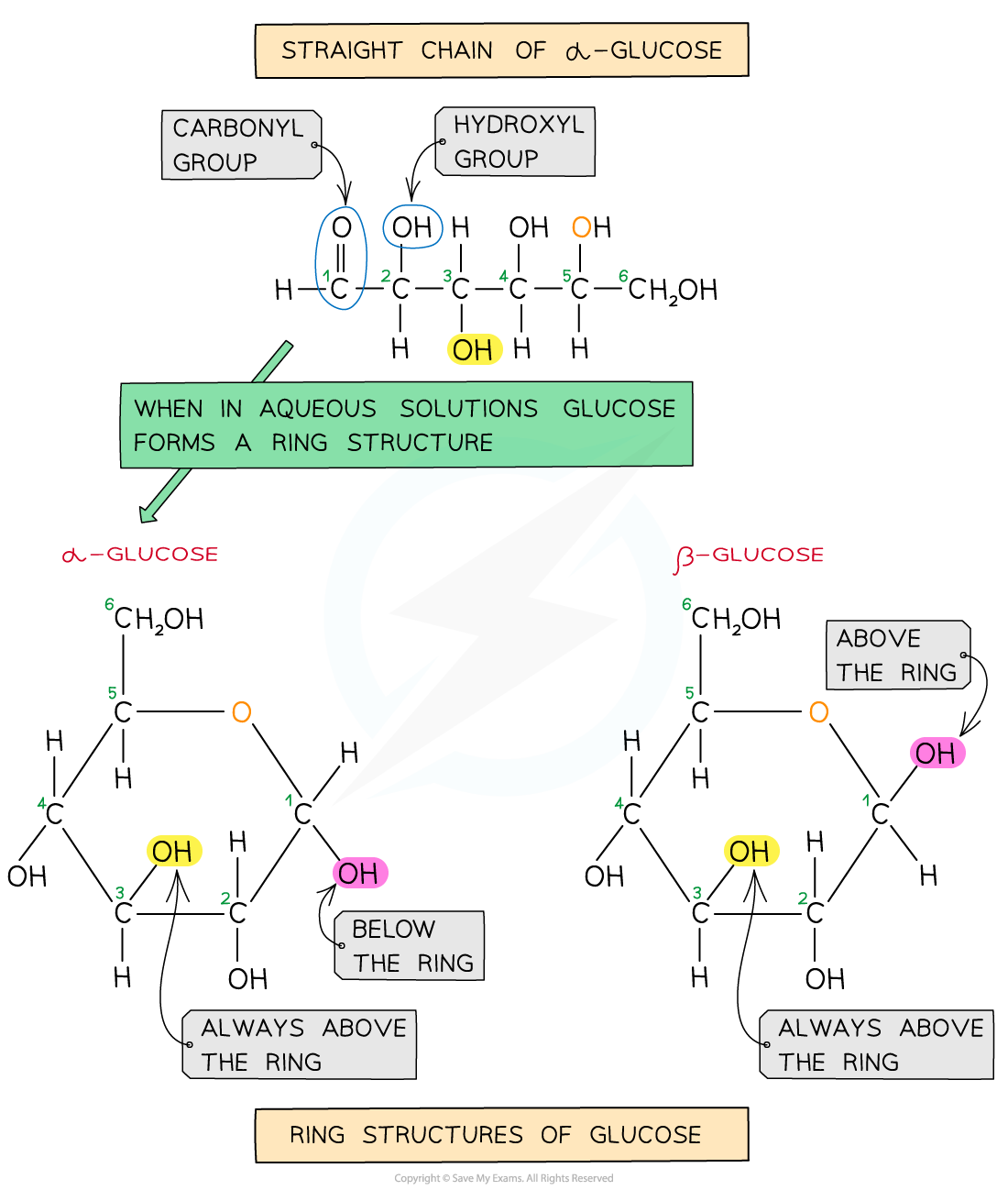
A comparison of the structure of alpha-glucose and beta-glucose
- Alpha-glucose and beta-glucose are isomers
- This seemingly minor example of isomerism has far-reaching consequences on the functions of the polymers
- It means that in order to form a glycosidic bond with a molecule of β-glucose, the next molecule of β-glucose in the chain must invert itself
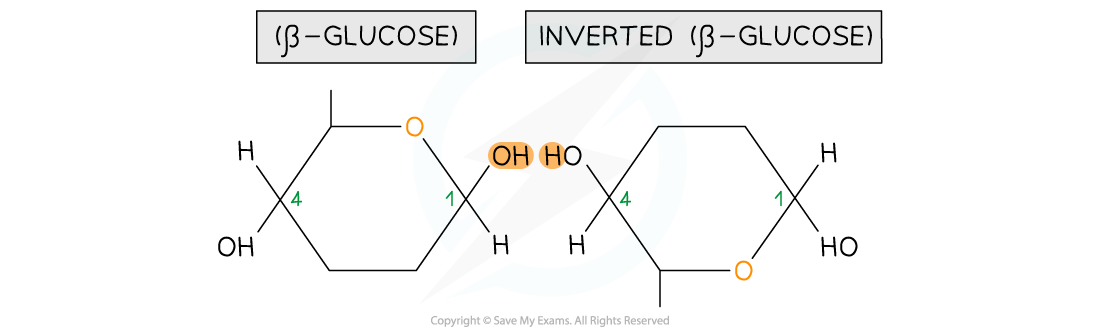
Two beta-glucose molecules orientation in a position where they are able to bond to each other
- This results in a chain of repeatedly inverted β-glucose monomers
- The alternating pattern of the monomers allows the chain to grow in long, straight lengths which gives great fibrous strength
- Hydrogen bonding occurs between strands of β-glucose monomers, adding strength to the polymer
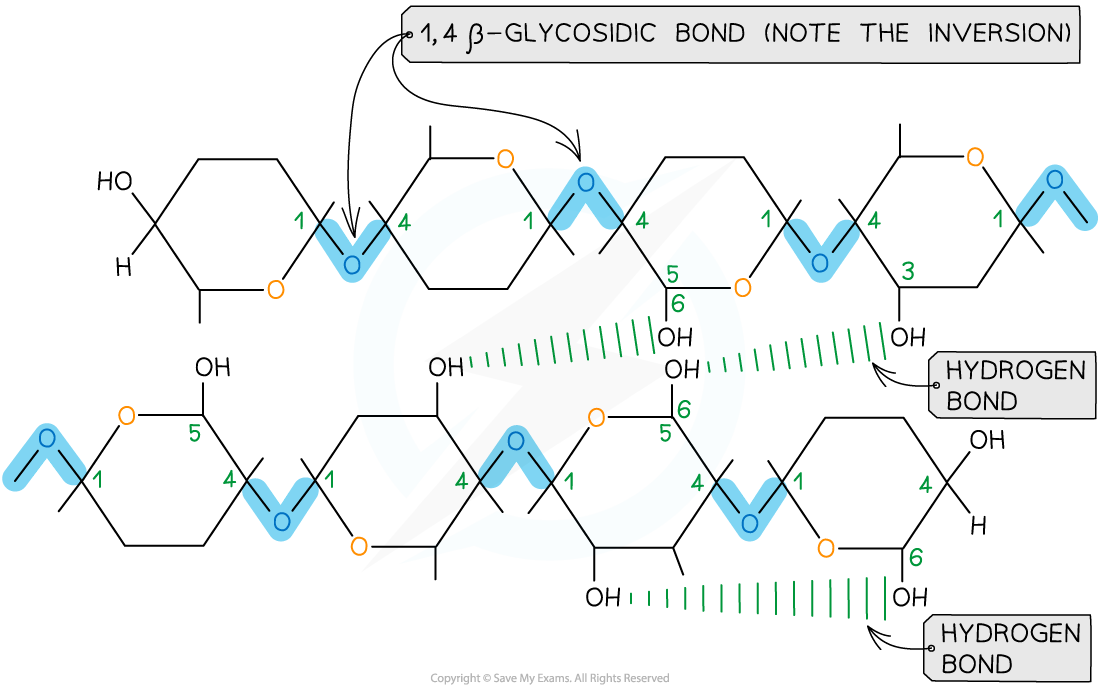
The alternating pattern of glycosidic bonds in cellulose
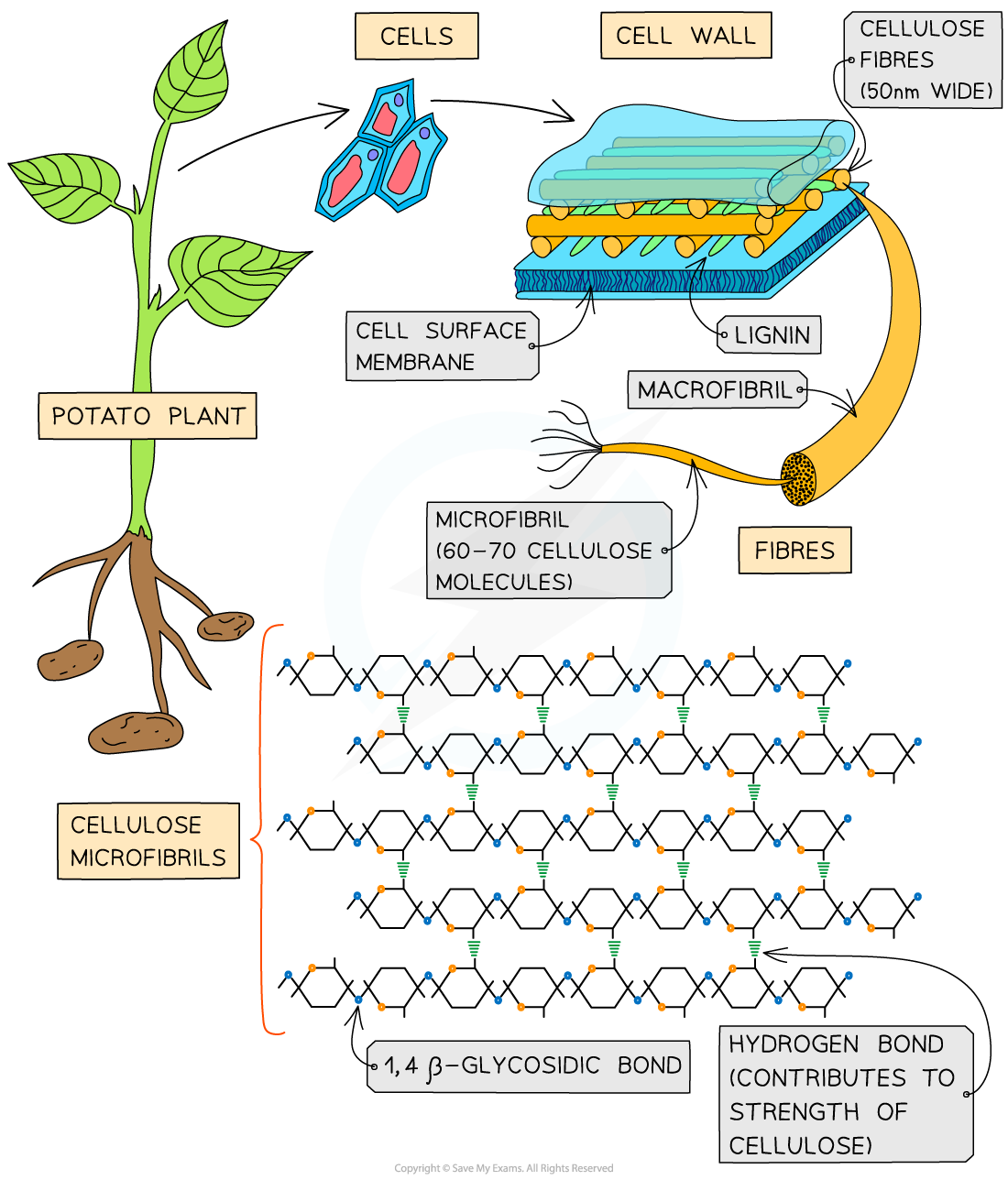
How cellulose fibres band together to provide plant strength
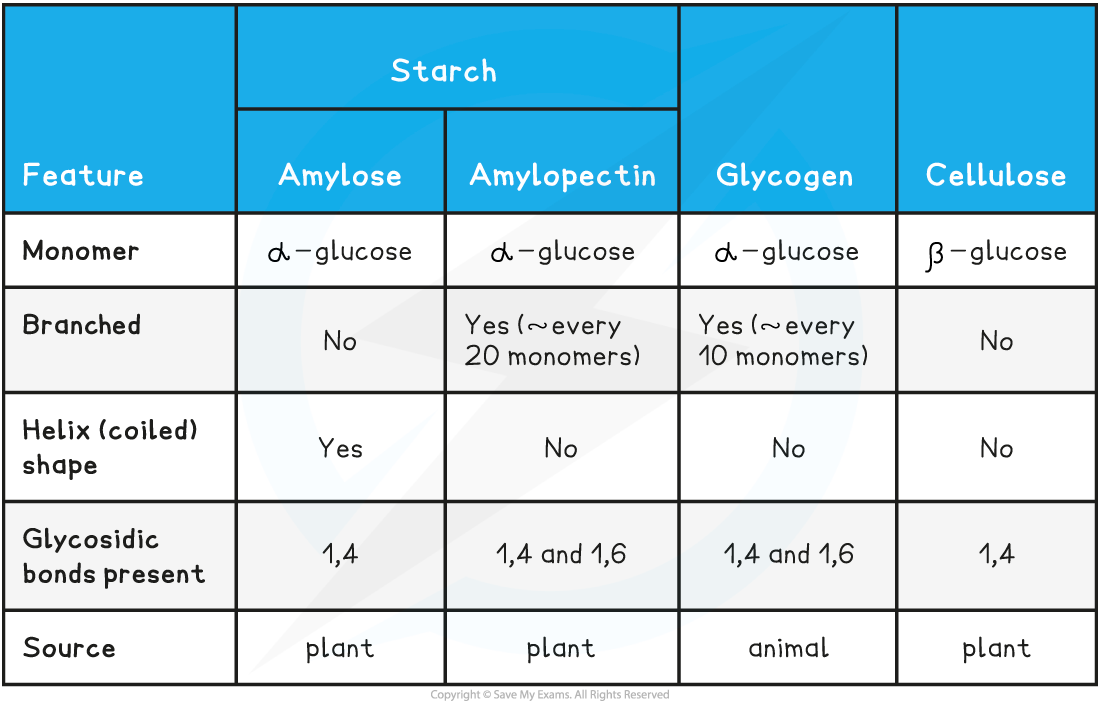
Summary of Polysaccharides Table
Exam Tip
Be clear about the differences between starch (amylose and amylopectin), cellulose and glycogen.
