Amphipathic Properties
Phospholipids
- Phospholipids form the basic structure of the membrane (the phospholipid bilayer)
- They are formed by a hydrophilic phosphate head bonding with two hydrophobic hydrocarbon (fatty acid) tails
- As phospholipids have a hydrophobic and hydrophilic part they are known as amphipathic
- The phosphate head of a phospholipid is polar (hydrophilic) and therefore soluble in water
- The fatty acid tail of a phospholipid is nonpolar (hydrophobic) and therefore insoluble in water
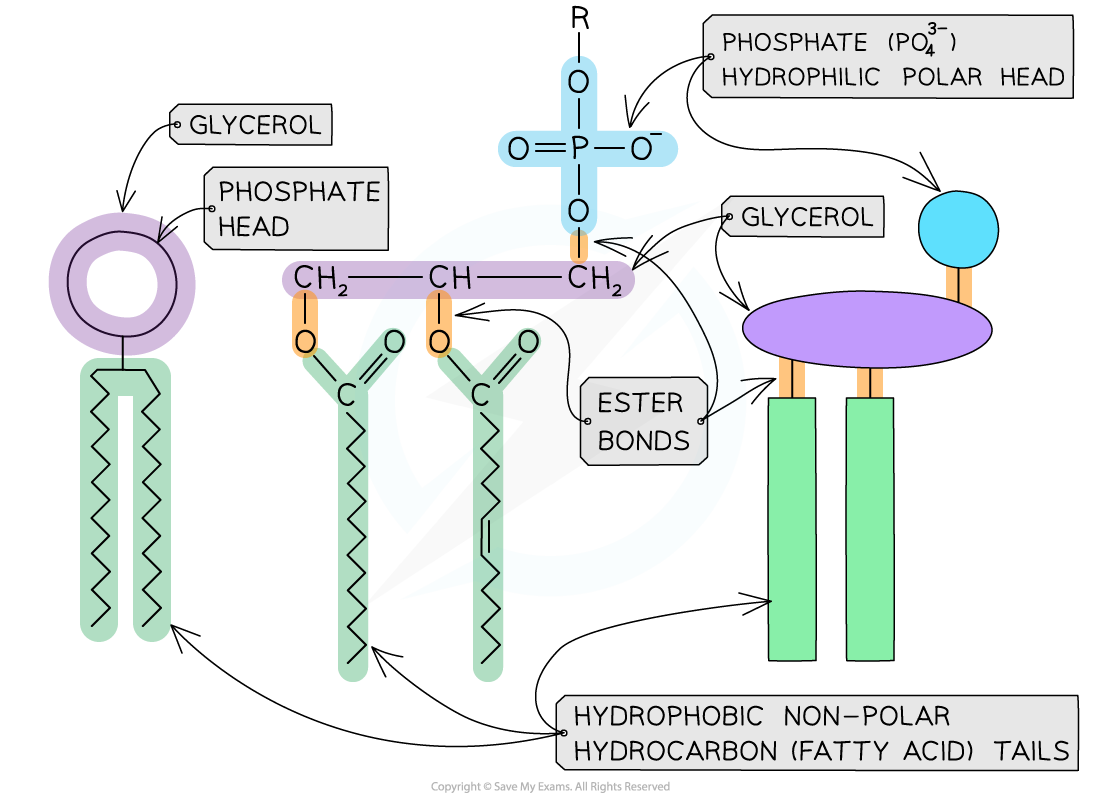
The generalised molecular structure of a phospholipid
- Due to their amphipathic properties, phospholipids display an emergent property when placed into water
- The hydrophilic phosphate heads orientate towards the water and the hydrophobic hydrocarbon tails orientate inwards (away from the water)
- They form a phospholipid monolayer
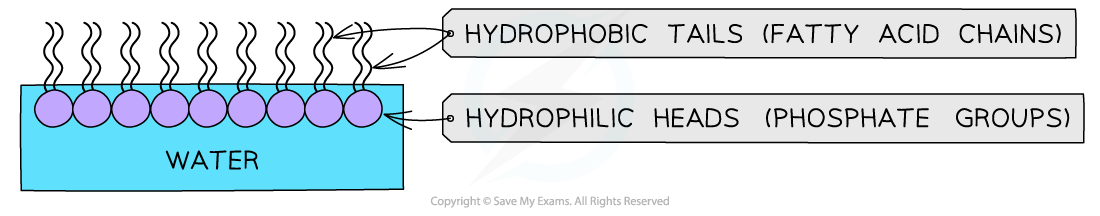
A phospholipid monolayer
- If phospholipids are mixed/shaken with water they form spheres with the hydrophilic phosphate heads facing out towards the water and the hydrophobic fatty acid tails facing inwards
- This is called a micelle
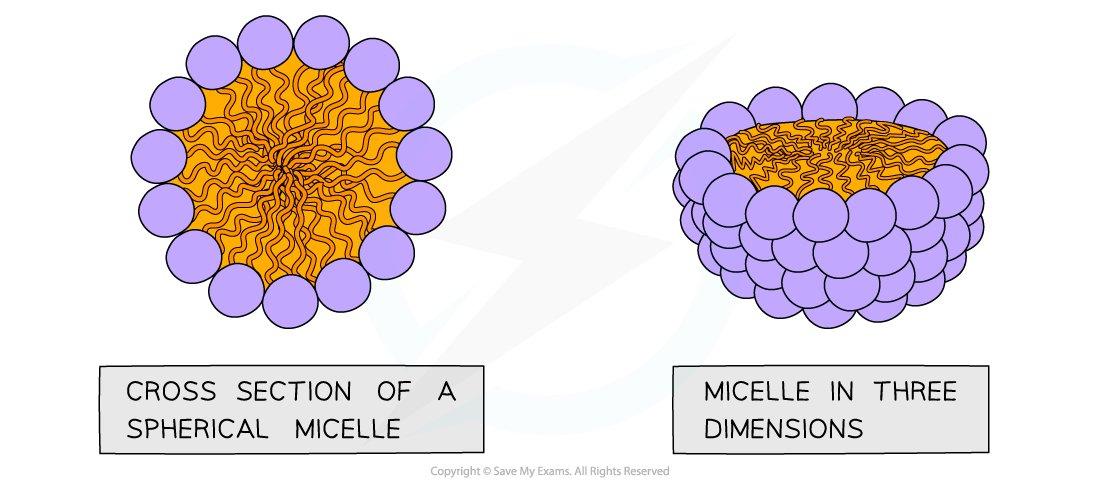
A micelle
- Alternatively, when there is a sufficient concentration of phospholipids present then two-layered structures may form
- These sheets are called phospholipid bilayers – this is the basic structure of the cell membrane
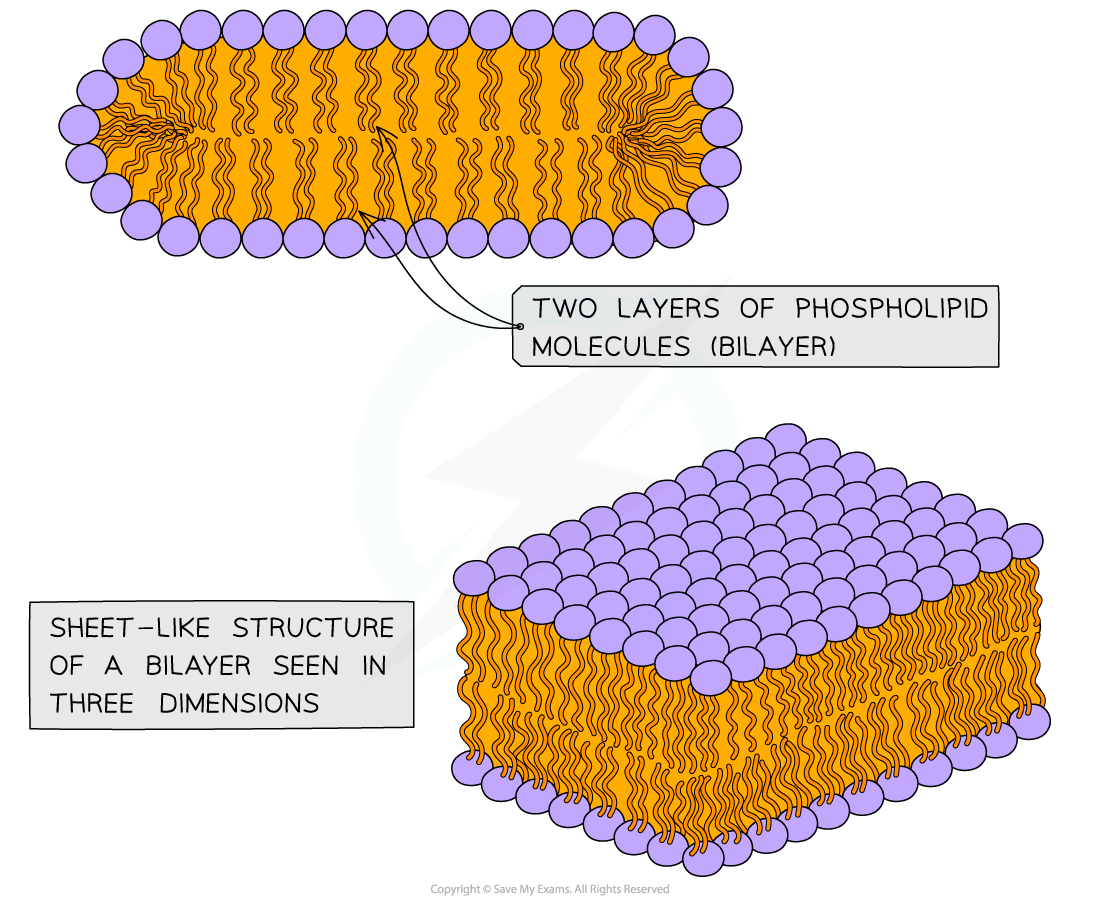
A phospholipid bilayer is composed of two layers of phospholipids; their hydrophobic tails facing inwards and hydrophilic heads outwards
- The two layers of phospholipids are loosely held together by weak hydrophobic interactions between the hydrocarbon tails allowing some membrane fluidity
- The amphipathic properties result in the phospholipid bilayer acting as a barrier to most water-soluble substances (the non-polar fatty acid tails prevent polar molecules or ions from passing across the membrane)
- This ensures water-soluble molecules such as sugars, amino acids and proteins cannot leak out of the cell and unwanted water-soluble molecules cannot get in
Animal Cell Membranes: Cholesterol
- Phospholipids and cholesterol are the two main components of animal cell plasma membranes (cholesterol is absent in plant membranes)
Cholesterol
- Cholesterol is a lipid that belongs to the steroid group
- It is amphipathic, with the majority of the cholesterol molecule being hydrophobic and therefore attracted to the hydrophobic hydrocarbon tails of the phospholipid
- The hydroxyl group of the cholesterol molecule is hydrophilic. It is attracted to the phosphate heads of the phospholipid
- Therefore in the plasma membrane cholesterol is positioned between phospholipids
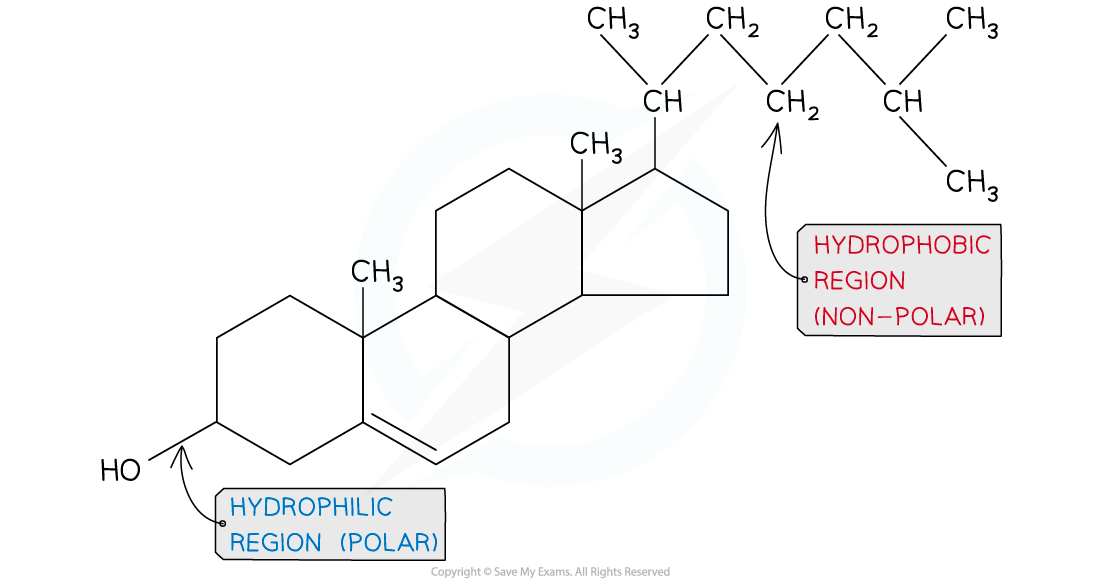
The molecular structure of cholesterol
Mammalian Membranes: Role of Cholesterol
- The plasma membrane is fluid, meaning the components are free to move
- The fluidity of the membrane needs to be controlled:
- If it was too fluid the cell could not regulate what moved in and out
- If it was not fluid enough then the cell would not be able to move and substances would be unable to move into or out of the cell
- Cholesterol helps with the regulation of the membrane fluidity and permeability
- Interaction between cholesterol and phospholipid tails stabilises the plasma membrane at higher temperatures by stopping the membrane from becoming too fluid
- Cholesterol molecules bind to the hydrophobic tails of phospholipids, stabilising them and causing phospholipids to pack more closely together
- Interaction between cholesterol and phospholipid tails stabilises the plasma membrane at higher temperatures by stopping the membrane from becoming too fluid
- At colder temperatures cholesterol increases the fluidity of the membrane, stopping it crystallizing and becoming too rigid
- This occurs because cholesterol stops the phospholipid tails packing too closely together
- The impermeability of the membrane to hydrophilic ions (e.g. sodium and hydrogen) is also reduced by cholesterol
Exam Tip
It is important to remember that cholesterol affects membrane fluidity and the permeability of hydrophilic ions (e.g. sodium and hydrogen) in mammal membranes.