Question 1
The image below shows a simplified version of the binding energy per nucleon E of nuclei versus the nucleon number N. Which of the following positions represents nuclei that are the most stable?

The image below shows a simplified version of the binding energy per nucleon E of nuclei versus the nucleon number N. Which of the following positions represents nuclei that are the most stable?

The rest mass of a nucleus of Boron-11 () can be considered as mB. The rest-masses of a neutron and proton can be considered as mN and mP respectively. Which of the following equations is the correct representation for the binding energy of Boron-11?
(5mP + 6mN – mB)c2
(5mP + 6mN + mB)c2
(5mP + 11mN – mB)c2
(6mP + 5mN – mB)c2
Which statement about nuclear binding energy is correct?
It is the energy equivalent of the mass of the neutrons in a nucleus
It is the energy required to separate nucleons in a nucleus
It is the energy required to overcome the electrostatic force between nucleons in the nucleus
It is the energy required to remove a single nucleon from a nucleus
The binding energy per nucleon is 7.98 MeV for an atom of . Approximately how much energy would be needed to completely separate the nucleons of this atom?
33.2 MeV
63.9 MeV
88.5 MeV
127.7 MeV
The mass defect for Helium-4 is 5.04 × 10–29 kg. What is the binding energy of Helium-4 closest to?
0.02 MeV
28 MeV
190 MeV
1225 MeV
Which of the following isotopes releases the least amount of potential energy during nuclear fission?
Uranium-235
Thorium-231
Radon-222
Osmium-190
Two identical nuclei of mass m fuse to form a single heavier nucleus (with no other products) with mass M. Which of the following statements is correct?
m = M
2m = M
2m > M
2m < M
Alchemists investigated the process of transmutation of mercury into gold. This can be represented by the following equation:
The sum of the rest masses of deuterium and mercury is 202.60 u and the sum of the rest masses of gold and helium are 200.97 u.
Take the energy equivalent of 0.001 u to be 1 MeV.
Which of the following can be determined from the information provided?
Energy of approximately 2000 MeV has been converted to a mass of 2 u
The kinetic energy of the products exceeds the kinetic energy of the reactants by 2000 MeV
The number of nuclei of gold is not equal to the number of nuclei of mercury
The kinetic energy of the deuterium nucleus was 2000 MeV
Nitrogen-14 can be transformed into Oxygen-17 by bombardment with high energy alpha particles, as described in the nuclear reaction equation below:
The total rest mass of the reactants is 18.006 u and total rest mass of the products is 18.007 u.
Which of the following statements about this reaction is correct?
A mass of 0.001 u has been converted to about 1 MeV of energy
The kinetic energy of the products exceeds the kinetic energy of the reactants by about 1 MeV
The kinetic energy of the reactants exceeds the kinetic energy of the products by about 1 MeV
The mass defect of this reaction is 0.002 u
The graph below shows how the average binding energy per nucleon varies with nucleon number for stable nuclei.
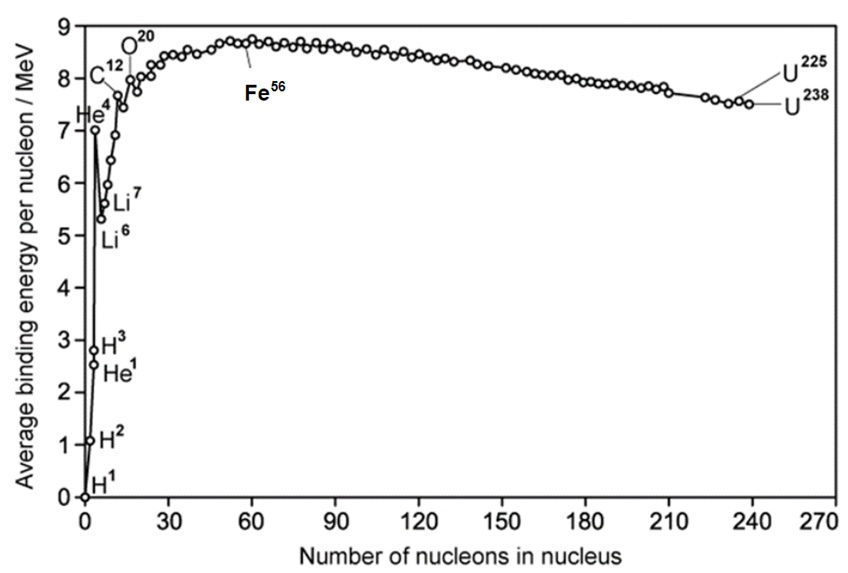
Approximately how much energy is released when the nucleus forms?
7.90 MeV
1430 MeV
533 MeV
888 MeV