Which of the following is the best definition of the unified atomic mass unit?
A unit of mass which is equal to the mass of one-twelfth of a neutral carbon-12 atom
A unit of mass which is equal to the mass of half of a carbon-12 atom
A unit of mass which is equal to the mass of twelve grams of a neutral carbon-12 atom
A unit of mass which is equal to twice the mass of a neutral carbon-12 atom
Choose your answer A B C D
View Answer
Next Question
Energy-mass equivalence is given by Δ E = Δ mc 2 .
Using the given equation, determine which of the following is a valid unit of mass.
Choose your answer A B C D
View Answer
Previous Question Next Question
The average binding energy per nucleon of Neon−20
What is the total energy required to separate the nucleons of one nucleus of
Choose your answer A B C D
View Answer
Previous Question Next Question
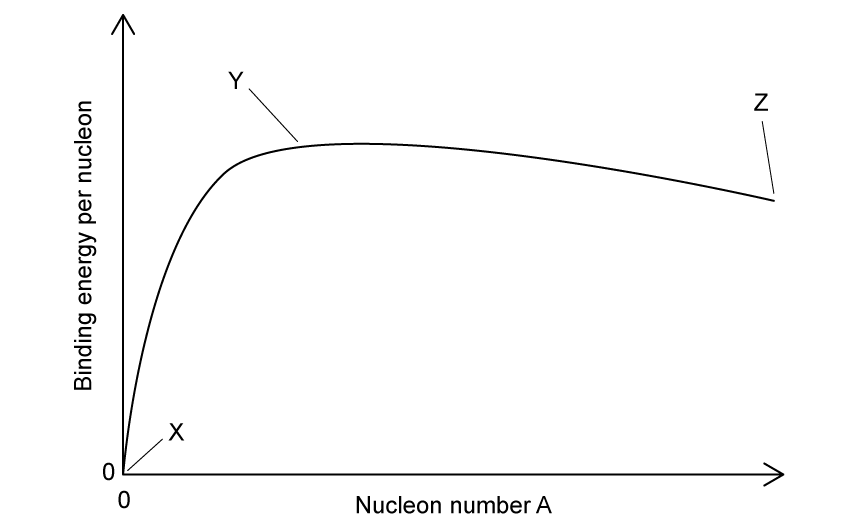
The graph shows the binding energy per nucleon against nucleon number.
Which row in the table gives possible elements found on the graph at positions X, Y and Z?
X
Y
Z
A. Uranium
Calcium
Xenon
B. Hydrogen
Uranium
Iron
C. Calcium
Hydrogen
Iron
D. Hydrogen
Iron
Uranium
Choose your answer A B C D
View Answer
Previous Question Next Question
Which statement is correct regarding nuclear fission?
The daughter nucleus has a greater nucleon number than the original nucleus
Energy is absorbed during nuclear fission
The combined mass of the daughter nuclei is less than the mass of the original nucleus
Nuclear fission is the joining of two small nuclei to produce a larger nucleus
Choose your answer A B C D
View Answer
Previous Question Next Question
What is the approximate mass of oxygen−16
Choose your answer A B C D
View Answer
Previous Question Next Question
Nuclear reactions can be represented by equations.
Which type of reaction does the equation show?
Choose your answer A B C D
View Answer
Previous Question Next Question
A nuclide of deuterium
Which statement is not correct about nuclear fusion?
For fusion to occur both nuclei must have high kinetic energy
The process of fusion absorbs energy
Fusion is the combining of two smaller nuclei into a larger nucleus
Fusion is the process that powers stars
Choose your answer A B C D
View Answer
Previous Question Next Question
Which statement is a definition of binding energy per nucleon?
The difference between an atom's mass and the sum of the masses of its nucleons
The binding energy of a nucleus divided by the number of nucleons in the nucleus
The energy required to break a nucleus into its constituent protons and neutrons
The amount of kinetic energy required for fusion to occur
Choose your answer A B C D
View Answer
Previous Question Next Question
The following fusion reaction occurs in stars:
The binding energies are given as follows:
The binding energy of deuterium,
The binding energy of tritium,
The binding energy of helium,
Given that the energy released is the difference between the binding energy of the products and the reactants, how much energy is released in this fusion process?
Choose your answer A B C D
View Answer
Previous Question