DP Chemistry Questionbank

Topic 4: Chemical bonding and structure
Description
[N/A]Directly related questions
-
16N.3.hl.TZ0.22c:
(i) Uranium hexafluoride, UF6, is used in the uranium enrichment process that produces fuel for nuclear reactors.
State the molecular shape of uranium hexafluoride.
(ii) Explain why uranium dioxide, UO2, has a very high melting point whereas uranium hexafluoride vapourises easily into gas.
-
20N.1.sl.TZ0.12:
Which series shows the correct order of metallic bond strength from strongest to weakest?
A.
B.
C.
D.
-
20N.1.hl.TZ0.9:
Which of these species contains the shortest carbon to oxygen bond length?
A.
B.
C.
D.
- 20N.2.hl.TZ0.4d(v): Describe the bonding in metals.
- 20N.3.sl.TZ0.1c: Non-polar solvents can be toxic. Suggest a modification to the experiment which allows the...
- 17M.1.sl.TZ1.9: A substance has the following properties: What is the most probable structure of this...
- 17M.1.sl.TZ1.24: What is the order of increasing boiling point? A. C4H10 < CH3COOH < CH3CH2CHO <...
- 17M.1.hl.TZ1.12: Which combination describes the bonding and structure in benzoic acid, C6H5COOH?
-
17M.2.sl.TZ1.2a:
Describe the bonding in metals.
-
17M.3.sl.TZ1.6a:
Determine the type of bond present in SbBr3, showing your method. Use sections 8 and 29 of the data booklet.
- 17M.1.sl.TZ2.11: What are the approximate bond angles and structure of crystalline SiO2?
-
17M.2.hl.TZ1.5a:
Estimate the H−N−H bond angle in methanamine using VSEPR theory.
-
17N.2.hl.TZ0.3b:
Explain why the melting points of the group 1 metals (Li → Cs) decrease down the group whereas the melting points of the group 17 elements (F → I) increase down the group.
-
17N.2.hl.TZ0.4a:
Draw the Lewis (electron dot) structures of PF3 and PF5 and use the VSEPR theory to deduce the molecular geometry of each species including bond angles.
-
17N.3.sl.TZ0.7b.i:
Describe how the structures of LDPE and HDPE affect one mechanical property of the plastics.
- 21M.1.sl.TZ1.24: Which series is in order of increasing boiling point? A. CH2CH2CH3OH CH3COCH3 ...
-
21M.1.sl.TZ2.11:
What is the formula of the compound formed from Ca2+ and PO43−?
A. CaPO4
B. Ca3(PO4)2
C. Ca2(PO4)3
D. Ca(PO4)2
-
21M.2.sl.TZ1.2a(i):
Draw the Lewis (electron dot) structure of hydrogen sulfide.
- 21M.2.hl.TZ1.3a: Deduce the ratio of Fe2+:Fe3+ in Fe3O4.
- 21M.2.hl.TZ1.1d(i): Describe the bonding in this type of solid.
-
18M.2.sl.TZ1.1e.i:
Suggest one reason why urea is a solid and ammonia a gas at room temperature.
- 18M.1.sl.TZ2.9: What is the formula of magnesium nitride? A. MgN B. Mg2N3 C. Mg3N D. Mg3N2
- 21N.1.sl.TZ0.11: Which compound contains both ionic and covalent bonds? A. CH3COONa B. CH3COOH C. K2O D. ...
-
21N.2.hl.TZ0.6c(v):
Suggest a reason that the Winkler Method used to measure biochemical oxygen demand (BOD) must be done at constant temperature.
- 22M.1.sl.TZ1.9: A compound consists of the ions Ca2+ and PO43–. What are the name and formula of the compound?
- 18N.1.sl.TZ0.10: How many lone pairs and bonding pairs of electrons surround the central chlorine atom in...
-
22M.1.sl.TZ2.12:
What is the main interaction between liquid CH4 molecules?
A. London (dispersion) forces
B. Dipole–dipole forces
C. Hydrogen bonding
D. Covalent bonding
- 22M.1.hl.TZ2.9: In which of the following compounds does ionic bonding predominate? A. HCl B. NaF C. ...
-
22M.2.sl.TZ1.2b:
Draw the Lewis (electron dot) structure of the ammonia molecule.
-
19M.1.hl.TZ1.9:
What is the order of increasing boiling point?
A. CH3CH2CH2CH3 < CH3CH(OH)CH3 < CH3COCH3 < CH3CO2H
B. CH3CH2CH2CH3 < CH3COCH3 < CH3CH(OH)CH3 < CH3CO2H
C. CH3CO2H < CH3COCH3 < CH3CH(OH)CH3 < CH3CH2CH2CH3
D. CH3CH2CH2CH3 < CH3COCH3 < CH3CO2H < CH3CH(OH)CH3
-
19M.2.sl.TZ1.2c:
Suggest how benzoic acid, Mr = 122.13, forms an apparent dimer, Mr = 244.26, when dissolved in a non-polar solvent such as hexane.
-
19M.2.sl.TZ2.1b(i):
Deduce the Lewis (electron dot) structure of ethyne.
- 19M.1.sl.TZ2.12: Which compound has hydrogen bonds between its molecules? A. CH4 B. CH4O C. CH3Cl D. CH2O
- 19N.2.hl.TZ0.1c: Predict the bond angle in the ozone molecule.
- 19N.1.sl.TZ0.10: Which compound has the shortest C to O bond? A. CH3CHO B. CO C. CO2 D. C2H5OC2H5
- 16N.3.sl.TZ0.18b: Suggest why isolation of the crude product involved the addition of ice-cold water.
-
16N.2.hl.TZ0.2d:
Draw the Lewis (electron dot) structure of the ethanedioate ion, –OOCCOO–.
-
16N.2.sl.TZ0.2d:
The Lewis (electron dot) structure of the ethanedioate ion is shown below.
Outline why all the C–O bond lengths in the ethanedioate ion are the same length and suggest a value for them. Use section 10 of the data booklet.
- 20N.2.sl.TZ0.4c: Discuss, referring to intermolecular forces present, the relative volatility of propanone and...
- 20N.2.hl.TZ0.4d(vi): Nickel alloys are used in aircraft gas turbines. Suggest a physical property altered by the...
-
20N.3.hl.TZ0.4c(i):
Alloying metals changes their properties. Suggest one property of magnesium that could be improved by making a magnesium–CNT alloy.
-
17M.2.sl.TZ1.4a:
Estimate the H−N−H bond angle in methanamine using VSEPR theory.
- 17M.1.sl.TZ2.12: Which metal has the strongest metallic bond? A. Li B. Na C. K D. Rb
-
17M.2.sl.TZ2.4b:
Deduce the Lewis (electron dot) structures of ozone.
-
17M.3.sl.TZ2.8a:
Explain which one of these fatty acids has the highest boiling point.
-
17N.2.hl.TZ0.4b:
Predict whether the molecules PF3 and PF5 are polar or non-polar.
-
17N.2.sl.TZ0.3b:
Predict with a reason, whether the molecule PF3 is polar or non-polar.
-
21M.2.sl.TZ2.2c:
Deduce the Lewis (electron dot) structure and molecular geometry of sulfur dichloride, SCl2.
-
18M.3.sl.TZ2.1a.i:
Graphene is two-dimensional, rather than three-dimensional, material.
Justify this by using the structure of graphene and information from the table.
-
18M.3.sl.TZ2.1a.ii:
Show that graphene is over 1600 times stronger than graphite.
-
18M.3.sl.TZ2.1a.iii:
Identify a value from the table which can be used to support the information about graphene given below.
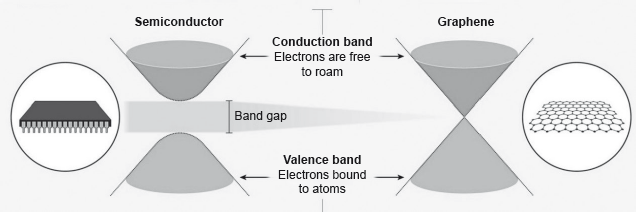
Electrons in a solid are restricted to certain ranges, or bands, of energy (vertical axis). In an insulator or semiconductor, an electron bound to an atom can break free only if it gets enough energy from heat or a passing photon to jump the “band gap”, but in graphene the gap is infinitely small.

- 21N.1.sl.TZ0.9: Which molecule has the weakest nitrogen to nitrogen bond? A. N2 B. N2H2 C. N2H4 D.
- 21N.2.sl.TZ0.3b(i): Deduce the electron domain and molecular geometry using VSEPR theory, and estimate the Cl–P–Cl...
- 22M.2.hl.TZ1.5a(ii): Compound A and Compound B are both liquids at room temperature and pressure. Identify the...
- 22M.2.sl.TZ2.3d(ii): Explain the electron domain geometry of NO3−.
-
22M.2.hl.TZ2.5c:
Describe the bonding in iron, Fe (s).
-
19M.2.hl.TZ1.2h:
Suggest how benzoic acid, Mr = 122.13, forms an apparent dimer, Mr = 244.26, when dissolved in a non-polar solvent such as hexane.
-
19M.2.hl.TZ2.1d(iii):
Explain why product B is water soluble.
- 19M.1.hl.TZ2.10: Which combination causes the strength of metallic bonding to increase?
-
19M.2.sl.TZ1.3a(i):
Describe the structure and bonding in solid sodium oxide.
-
19M.2.sl.TZ2.1b(ii):
Compare, giving a reason, the length of the bond between the carbon atoms in ethyne with that in ethane, C2H6.
-
19M.2.sl.TZ2.1d(iii):
Explain why product B is water soluble.
-
19M.1.sl.TZ1.9:
What is the order of increasing boiling point?
A. CH3CH2CH2CH3 < CH3CH(OH)CH3 < CH3COCH3 < CH3CO2H
B. CH3CH2CH2CH3 < CH3COCH3 < CH3CH(OH)CH3 < CH3CO2H
C. CH3CO2H < CH3COCH3 < CH3CH(OH)CH3 < CH3CH2CH2CH3
D. CH3CH2CH2CH3 < CH3COCH3 < CH3CO2H < CH3CH(OH)CH3
- 19M.1.sl.TZ1.12: Which combination corresponds to a strong metallic bond?
- 19M.1.sl.TZ1.11: Which describes an ionic compound?
- 19N.2.sl.TZ0.1b: Outline why both bonds in the ozone molecule are the same length and predict the bond length in...
- 20N.2.hl.TZ0.2a: Predict the electron domain and molecular geometries around the oxygen atom of molecule A using...
- 20N.3.sl.TZ0.4b(i): Alloying metals changes their properties. Suggest one property of magnesium that could be...
-
20N.3.sl.TZ0.6b(ii):
State the most significant intermolecular forces in the phospholipid in b(i).
-
17M.2.sl.TZ1.2e.ii:
A chloride of titanium, TiCl4, melts at 248 K. Suggest why the melting point is so much lower than that of KCl.
-
17M.2.hl.TZ1.2d.ii:
Suggest why the melting point of vanadium is higher than that of titanium.
-
17M.3.sl.TZ1.19a:
Suggest why aspirin is slightly soluble in water. Refer to section 37 of the data booklet.
-
17M.2.hl.TZ2.4b.ii:
Deduce one resonance structure of ozone and the corresponding formal charges on each oxygen atom.
-
17N.1.sl.TZ0.10:
Which compound has the shortest C–N bond?
A. CH3NH2
B. (CH3)3CNH2
C. CH3CN
D. CH3CHNH
- 21M.2.hl.TZ1.1f: Explain why the addition of small amounts of carbon to iron makes the metal harder.
-
21M.2.hl.TZ1.7a(i):
Draw a Lewis (electron dot) structure for ozone.
- 21M.2.sl.TZ2.2d: Suggest, giving reasons, the relative volatilities of SCl2 and H2O.
- 21M.2.hl.TZ2.2d: Suggest, giving reasons, the relative volatilities of SCl2 and H2O.
-
18M.2.sl.TZ1.2a:
Describe the nature of ionic bonding.
-
18M.3.sl.TZ1.1a.i:
Part of this molecule is hydrophilic (bonds readily to water) and part hydrophobic (does not bond readily to water). Draw a circle around all of the hydrophilic part of the molecule.
-
18M.3.sl.TZ2.1b:
Diamond, graphene, and graphite are all network solids.
Suggest, giving a reason, the electron mobility of diamond compared to graphene.
- 21N.1.sl.TZ0.12: The following compounds have similar relative molecular masses. What is the order of increasing...
- 21N.1.sl.TZ0.13: Which alcohol is least soluble in water? A. CH3OH B. CH3CH2OH C. CH3CH2CH2OH D. ...
- 21N.2.hl.TZ0.3b(i): Deduce the electron domain and molecular geometry using VSEPR theory, and estimate the Cl–P–Cl...
- 18N.2.sl.TZ0.6b: Explain why butanoic acid is a liquid at room temperature while ethylamine is a gas at room...
- 22M.2.sl.TZ2.3d(i): Draw the Lewis structure of NO3−.
- 22M.2.hl.TZ2.6c(i): Draw the Lewis structure of SO3.
-
19M.2.hl.TZ1.5c(iv):
State the type of bond formed when chloramine is protonated.
-
19M.2.hl.TZ1.5c(iii):
Deduce the molecular geometry of chloramine and estimate its H–N–H bond angle.
Molecular geometry:
H–N–H bond angle:
-
19M.2.sl.TZ2.1b(iii):
Identify the type of interaction that must be overcome when liquid ethyne vaporizes.
- 19N.2.sl.TZ0.6a(iv): Suggest why water vapour deviates significantly from ideal behaviour when the gases are cooled,...
- 19N.3.sl.TZ0.4b: Predict, with a reason, whether isotactic or atactic polypropene has the higher melting point.
- 19N.2.sl.TZ0.1a: Draw the Lewis structures of oxygen, O2, and ozone, O3.
-
16N.2.sl.TZ0.1c:
Explain why the boiling point of ethane-1,2-diol is significantly greater than that of ethene.
- 16N.1.sl.TZ0.9: Which pair of molecules has the same bond angles? A. PCl3 and BCl3 B. SO2 and CO2 C. H2O...
-
16N.3.sl.TZ0.6c:
(i) Suggest why incomplete combustion of plastic, such as polyvinyl chloride, is common in industrial and house fires.
(ii) Phthalate plasticizers such as DEHP, shown below, are frequently used in polyvinyl chloride.
With reference to bonding, suggest a reason why many adults have measurable levels of phthalates in their bodies.
- 16N.1.sl.TZ0.12: Which substance has a giant covalent structure?
- 16N.2.sl.TZ0.4f: Describe the structure and bonding in solid magnesium oxide.
- 17M.1.sl.TZ1.12: Which correctly states the strongest intermolecular forces in the compounds below?
-
17M.1.sl.TZ2.19:
Which of the following does not react with dilute HCl(aq)?
A. Na2CO3
B. Cu
C. Zn
D. CuO
-
17M.2.sl.TZ2.1c.ii:
Explain the electrical conductivity of molten Na2O and P4O10.
-
17M.2.sl.TZ2.3b:
Deduce the Lewis (electron dot) structure and molecular geometry of PCl3.
-
17M.2.sl.TZ2.4a.ii:
State why hydrazine has a higher boiling point than dinitrogen tetraoxide.
-
17N.3.sl.TZ0.9c:
Explain how the inclusion of carbohydrates in plastics makes them biodegradable.
-
17N.3.hl.TZ0.7c:
Identify the type of intermolecular bonding that is responsible for Kevlar®’s strength.
- 21M.2.hl.TZ1.1d(v): Suggest why chemists find it convenient to classify bonding into ionic, covalent and metallic.
- 18M.1.hl.TZ1.11: Which metal has the strongest metallic bonding? A. Na B. Mg C. Al D. Ca
-
18M.2.hl.TZ1.2a:
Describe the nature of ionic bonding.
-
18M.2.hl.TZ1.1b:
The structural formula of urea is shown.
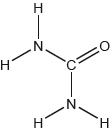
Predict the electron domain and molecular geometries at the nitrogen and carbon atoms, applying the VSEPR theory.
-
18M.2.hl.TZ2.7e:
Carbon and silicon are elements in group 14.
Explain why CO2 is a gas but SiO2 is a solid at room temperature.
- 18M.1.sl.TZ1.10: Which form of carbon is the poorest electrical conductor? A. Graphite B. Graphene C. ...
- 21N.1.sl.TZ0.10: Which combination would create the strongest ionic bond?
- 21N.2.sl.TZ0.3b(ii): Explain the polarity of PCl3.
- 22M.1.sl.TZ1.11: Which molecule is most polar? A. CF4 B. CCl4 C. CHF3 D. CClF3
- 18N.2.hl.TZ0.3c: Predict, using the VSEPR theory, the geometry of the BrO3− ion and the O−Br−O bond angles.
-
22M.2.sl.TZ1.1g:
State the types of bonding in magnesium, oxygen and magnesium oxide, and how the valence electrons produce these types of bonding.
-
22M.2.hl.TZ2.8a(i):
Outline two differences between the bonding of carbon atoms in C60 and diamond.
-
19M.2.hl.TZ1.5c(i):
Draw a Lewis (electron dot) structure of chloramine.
- 19M.1.hl.TZ1.11: Which combination corresponds to a strong metallic bond?
- 19M.1.hl.TZ2.9: How does a lithium atom form the most stable ion? A. The atom gains a proton to form a positive...
- 19M.1.sl.TZ2.10: Which combination causes the strength of metallic bonding to increase?
-
19N.3.sl.TZ0.17b:
Aspirin, C6H4(OCOCH3)COOH, is only slightly soluble in water.
Outline, including an equation, how aspirin can be made more water-soluble. Use section 37 in the data booklet.
- 19N.1.sl.TZ0.9: Which is correct for all solid ionic compounds? A. High volatility B. Poor electrical...
- 16N.3.sl.TZ0.18c: Justify the conclusion that recrystallization increased the purity of the product, by reference...
-
16N.3.sl.TZ0.8b:
The table below shows average figures for the percentage fatty acid composition of some common fats and oils.
(i) Deduce, with a reason, which fat or oil from the table above has the lowest iodine number.
(ii) Deduce, with a reason, which fat or oil from the table above is most likely to become rancid when exposed to the air.
(iii) The P/S index of a fat or oil is the ratio of polyunsaturated fat to saturated fat present. It is sometimes used to compare the relative health benefits of different lipids in the diet. Calculate the P/S index of beef fat and soybean oil.
(iv) Suggest why a P/S index of greater than 1 is considered beneficial to health.
(v) Cotton seed oil and corn oil have similar iodine numbers but the melting point of cotton seed oil is higher than that of corn oil. Suggest an explanation in terms of the structure and bonding in these two oils.
- 16N.1.sl.TZ0.11: Between which pair of molecules can hydrogen bonding occur? A. CH4 and H2OB. CH3OCH3 and CF4 C....
-
20N.1.sl.TZ0.10:
Which molecule is most polar?
A.
B.
C.
D.
- 20N.2.sl.TZ0.2a: Predict the electron domain and molecular geometries around the oxygen atom of molecule A using...
-
20N.3.sl.TZ0.5a(ii):
Proteins are polymers of amino acids.
The mixture is composed of glycine, , and isoleucine, . Their structures can be found in section 33 of the data booklet.
Deduce, referring to relative affinities and , the identity of A1.
- 20N.3.sl.TZ0.11b: Aspirin crystals are rinsed with water after recrystallization to remove impurities.Suggest why...
- 17M.1.sl.TZ1.10: Which two atoms form the most polar bond? A. C and F B. C and Cl C. Si and F D. ...
-
17M.1.sl.TZ1.11:
Which combination describes the sulfate(IV) ion, SO32– (also known as sulfite ion)?
-
17M.2.sl.TZ1.2d.ii:
Explain why an aluminium-titanium alloy is harder than pure aluminium.
-
17M.2.sl.TZ1.2e.i:
State the type of bonding in potassium chloride which melts at 1043 K.
-
17M.2.hl.TZ1.5b:
State the electron domain geometry around the nitrogen atom and its hybridization in methanamine.
-
17M.3.sl.TZ1.13d:
The solubility of a vitamin depends on its structure.
Identify the vitamin given in section 35 of the data booklet that is the most soluble in water.
- 17M.1.sl.TZ2.9: How many bonding electrons are there in the urea molecule? A. 8 B. 16 C. 20 D....
-
17M.1.sl.TZ2.10:
Which bonds cause the boiling point of water to be significantly greater than that of hydrogen sulfide?
A. London (dispersion)
B. Covalent
C. Ionic
D. Hydrogen
-
17M.2.sl.TZ2.4a.i:
State and explain the difference in bond strength between the nitrogen atoms in a hydrazine and nitrogen molecule.
-
17M.3.sl.TZ2.11:
Suggest, in terms of its structure, why vitamin D is fat-soluble using section 35 of the data booklet.
- 17N.1.sl.TZ0.11: Which of the following series shows increasing hydrogen bonding with water? A. Propane <...
- 21M.1.sl.TZ2.9: Which compound has the greatest volatility under the same conditions? A. SO2 B. SiO2 C. ...
- 21M.1.sl.TZ2.12: Which is the correct order based on increasing strength? A. covalent bonds < hydrogen bonds...
- 21M.2.sl.TZ1.1a: Outline why metals, like iron, can conduct electricity.
- 21M.2.hl.TZ2.2b(ii): Describe metallic bonding and how it contributes to electrical conductivity.
-
18M.2.hl.TZ1.1e.i:
Suggest one reason why urea is a solid and ammonia a gas at room temperature.
-
18M.3.sl.TZ1.1a.ii:
When a small amount of palmitic acid is placed in water it disperses to form a layer on the surface that is only one molecule thick. Explain, in terms of intermolecular forces, why this occurs.
-
18M.1.sl.TZ2.11:
What are the predicted electron domain geometries around the carbon and both nitrogen atoms in urea, (NH2)2CO, applying VSEPR theory?
-
18M.2.sl.TZ2.6a.i:
Explain why the hydrides of group 16 elements (H2O, H2S, H2Se and H2Te) are polar molecules.
-
21N.2.sl.TZ0.3a(i):
Sketch the Lewis (electron dot) structure of the P4 molecule, containing only single bonds.
-
21N.2.hl.TZ0.3a(i):
Sketch the Lewis (electron dot) structure of the P4 molecule, containing only single bonds.
- 18N.2.sl.TZ0.3c: Predict, using the VSEPR theory, the geometry of the BrO3− ion and the O−Br−O bond angles.
-
22M.1.sl.TZ2.9:
Which statement best describes the intramolecular bonding in HCN (l)?
A. Electrostatic attractions between H+ and CN− ions
B. Hydrogen bonding
C. Van der Waals forces and hydrogen bonding
D. Electrostatic attractions between pairs of electrons and positively charged nuclei
-
22M.2.hl.TZ2.7c:
Suggest why hydrogen chloride, HCl, has a lower boiling point than hydrogen cyanide, HCN.
-
19M.2.hl.TZ2.1b(iii):
Identify the type of interaction that must be overcome when liquid ethyne vaporizes.
-
19M.2.hl.TZ2.3d(ii):
State, giving a reason, the shape of the dinitrogen monoxide molecule.
- 19N.3.sl.TZ0.10c: Explain why maltose, C12H22O11, is soluble in water.
- 19N.2.hl.TZ0.1a: Draw the Lewis structures of oxygen, O2, and ozone, O3.
-
16N.2.hl.TZ0.2e:
Outline why all the C–O bond lengths in the ethanedioate ion are the same length and suggest a value for them. Use section 10 of the data booklet.
- 16N.3.sl.TZ0.3b: Predict the predominant type of bonding for a binary compound AB in which the electronegativity...
- 20N.2.sl.TZ0.4d(iv): Nickel alloys are used in aircraft gas turbines. Suggest a physical property altered by the...
- 20N.2.hl.TZ0.4c: Discuss, referring to intermolecular forces present, the relative volatility of propanone and...
-
20N.3.hl.TZ0.6a(ii):
Proteins are polymers of amino acids.
The mixture is composed of glycine, , and isoleucine, . Their structures can be found in section 33 of the data booklet.
Deduce, referring to relative affinities and , the identity of A1.
-
17M.3.sl.TZ1.6b:
Lanthanum has a similar electronegativity to group 2 metals. Explain, in terms of bonding and structure, why crystalline lanthanum bromide is brittle.
-
17M.3.sl.TZ1.10b.ii:
Explain why the difference in their structures affects their melting points.
-
17M.2.hl.TZ1.5c:
Ammonia reacts reversibly with water.
Explain the effect of adding ions on the position of the equilibrium. - 21M.1.sl.TZ1.12: Along which series is the bond angle increasing? A. NH3 H2O CH4 B. CH4 NH3 H2O C. H2O ...
- 21M.2.sl.TZ1.1c(i): Describe the bonding in this type of solid.
- 21M.2.sl.TZ1.3a: Deduce the ratio of Fe2+:Fe3+ in Fe3O4.
-
21M.2.hl.TZ2.2c:
Deduce the Lewis (electron dot) structure and molecular geometry of sulfur tetrafluoride, SF4, and sulfur dichloride, SCl2.
-
18M.2.hl.TZ1.2d.ii:
Outline why solid calcium is a good conductor of electricity.
-
18M.1.sl.TZ1.9:
What is the formula of ammonium phosphate?
A. (NH3)3PO4
B. (NH4)3PO4
C. (NH4)2PO4
D. (NH3)2PO3
- 18M.1.sl.TZ1.11: What is the molecular geometry and bond angle in the molecular ion NO3−?
-
18M.1.sl.TZ1.12:
What are the strongest intermolecular forces between molecules of propanone, CH3COCH3, in the liquid phase?
A. London (dispersion) forces
B. Covalent bonding
C. Hydrogen bonding
D. Dipole–dipole forces
-
18M.2.sl.TZ1.1b:
The structural formula of urea is shown.
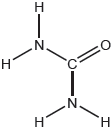
Predict the electron domain and molecular geometries at the nitrogen and carbon atoms, applying the VSEPR theory.
-
18M.2.sl.TZ1.1e.ii:
Sketch two different hydrogen bonding interactions between ammonia and water.
-
18M.2.sl.TZ1.2d.ii:
Outline why solid calcium is a good conductor of electricity.
- 18M.1.sl.TZ2.10: Which species has the longest carbon to oxygen bond length? A. CO B. CH3OH C. ...
-
18M.2.sl.TZ2.6a.ii:
The graph shows the boiling points of the hydrides of group 16 elements.
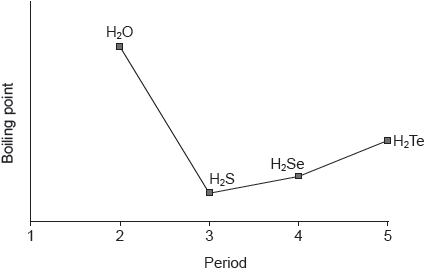
Explain the increase in the boiling point from H2S to H2Te.
- 22M.1.sl.TZ1.10: What is the explanation for the high melting point of sodium chloride? A. The covalent bond...
- 22M.1.sl.TZ1.12: For which species can resonance structures be drawn? A. HCOOH B. HCOO– C. CH3OH D. H2CO3
-
18N.2.sl.TZ0.3b:
Draw the Lewis (electron dot) structure for BrO3− that obeys the octet rule.
- 18N.1.sl.TZ0.11: Which compound has the highest boiling point? A. CH3CHO B. CH3CH2F C. CH3OCH3 D. ...
- 18N.2.hl.TZ0.8a: Suggest why the three-membered ring in methyloxirane is unstable.
-
18N.2.hl.TZ0.3b.i:
Draw two Lewis (electron dot) structures for BrO3−.
- 22M.2.sl.TZ1.3c(ii): Identify the strongest force between the molecules of Compound B.
-
22M.2.hl.TZ1.1g:
State the types of bonding in magnesium, oxygen and magnesium oxide, and how the valence electrons produce these types of bonding.
-
22M.2.hl.TZ1.6a(ii):
Deduce a Lewis (electron dot) structure of the nitric acid molecule, HNO3, that obeys the octet rule, showing any non-zero formal charges on the atoms.
-
19M.2.hl.TZ1.3c:
Describe the structure and bonding in solid sodium oxide.
-
19M.2.hl.TZ2.5d(i):
Identify the type of bonding in sodium hydrogencarbonate.
Between sodium and hydrogencarbonate:
Between hydrogen and oxygen in hydrogencarbonate:
-
19M.1.hl.TZ1.10:
What is the IUPAC name of NiCO3?
A. nickel(II) carbonate
B. nickel carbonate
C. nickel(I) carbonate
D. nitrogen(I) carbonate
- 19M.1.sl.TZ2.9: How does a lithium atom form the most stable ion? A. The atom gains a proton to form a positive...
- 19N.2.sl.TZ0.1d: Discuss how the different bond strengths between the oxygen atoms in O2 and O3 in the ozone layer...
-
19N.2.hl.TZ0.3d(ii):
Explain why the compound C3H8O, produced in (a)(iv), has a higher boiling point than compound C3H6O, produced in d(i).
- 19N.3.hl.TZ0.13b: Explain how the double-helical structure of DNA is stabilized once formed.
-
19N.2.sl.TZ0.3d(ii):
Explain why the compound C2H6O, produced in (b), has a higher boiling point than compound C2H4O, produced in d(i).
- 19N.2.hl.TZ0.1d: Discuss how the different bond strengths between the oxygen atoms in O2 and O3 in the ozone layer...
- 19N.3.hl.TZ0.15c: Explain why maltose, C12H22O11, is soluble in water.
-
20N.1.sl.TZ0.9:
Which formula is correct?
A.
B.
C.
D.
- 20N.2.sl.TZ0.4d(iii): Describe the bonding in metals.
- 20N.3.hl.TZ0.15b: Aspirin crystals are rinsed with water after recrystallization to remove impurities.Suggest why...
-
17M.3.sl.TZ1.11b.i:
The drain pipe of a kitchen sink can become clogged by fatty acids, such as linoleic acid, C18H32O2, but not by the trisaccharide, raffinose, C18H32O16, containing the same number of carbon atoms.
Explain why raffinose is far more water soluble than linoleic acid.
-
17M.3.sl.TZ2.16a:
Explain why diamorphine passes more readily than morphine through the blood-brain barrier.
- 17N.1.sl.TZ0.9: The electronegativity values of four elements are given. What is the order of increasing...
-
17N.2.sl.TZ0.2b:
Explain why the melting points of the group 1 metals (Li → Cs) decrease down the group.
-
17N.2.sl.TZ0.3a:
Draw the Lewis (electron dot) structures of PF3 and PF4+ and use the VSEPR theory to deduce the molecular geometry of each species.
- 21M.1.sl.TZ1.11: Which substance is most likely to be ionic?
- 21M.1.sl.TZ1.9: The Lewis structure of methylamine is shown. What is the molecular geometry around N? A. ...
-
21M.1.sl.TZ2.10:
Which compound has the shortest C to N bond?
A. HCN
B. CH3CH2NH2
C. CH3CHNH
D. (CH3)2NH
- 21M.1.hl.TZ2.12: Which atom has an expanded octet? A. C in CO2 B. S in SCl4 C. O in H2O2 D. P in PCl3
- 21M.2.sl.TZ1.1c(iv): Suggest why chemists find it convenient to classify bonding into ionic, covalent and metallic.
- 21M.2.sl.TZ1.1e: Explain why the addition of small amounts of carbon to iron makes the metal harder.
- 21M.2.sl.TZ1.2a(ii): Predict the shape of the hydrogen sulfide molecule.
-
18M.1.sl.TZ2.12:
The compounds shown below have similar relative molecular masses. What is the correct order of increasing boiling point?
A. CH3COOH < (CH3)2CO < (CH3)2CHOH
B. CH3COOH < (CH3)2CHOH < (CH3)2CO
C. (CH3)2CO < CH3COOH < (CH3)2CHOH
D. (CH3)2CO < (CH3)2CHOH < CH3COOH
-
18M.3.sl.TZ2.1c:
The melting point of diamond at 1 × 106 kPa is 4200 K (in the absence of oxygen).
Suggest, based on molecular structure, why graphene has a higher melting point under these conditions.
- 21N.2.hl.TZ0.3b(ii): Outline the reason why PCl5 is a non-polar molecule, while PCl4F is polar.
- 18N.1.sl.TZ0.12: Which molecule is polar? A. BeCl2 B. BCl3 C. NCl3 D. CCl4
- 18N.2.sl.TZ0.4b.ii: State a physical property of sodium oxide.
-
17M.3.hl.TZ1.25a:
Suggest why aspirin is slightly soluble in water. Refer to section 37 of the data booklet.
- 22M.1.hl.TZ1.13: What are the electron domain and molecular geometries of the XeF4 molecule?
-
22M.2.hl.TZ2.8a(ii):
Explain why C60 and diamond sublime at different temperatures and pressures.
-
19M.2.hl.TZ2.1b(ii):
Compare, giving a reason, the length of the bond between the carbon atoms in ethyne with that in ethane, C2H6.
-
19M.2.sl.TZ2.5b(iii):
Identify the type of bonding in sodium hydrogencarbonate.
Between sodium and hydrogencarbonate:
Between hydrogen and oxygen in hydrogencarbonate:
- 19N.1.sl.TZ0.11: Which describes a resonance structure? A. Double bond can be drawn in alternative...
-
19N.1.sl.TZ0.12:
What is the structure and bonding in SiO2 (s)?
-
19N.3.sl.TZ0.5b(i):
Determine the percentage of ionic bonding in alumina using sections 8 and 29 of the data booklet.
- 16N.1.hl.TZ0.11: How many electrons form the carbon–oxygen bond in methanal, HCHO? A. 2 B. 4 C. 8 D. 12
-
20N.1.sl.TZ0.11:
Which combination correctly describes the geometry of the carbonate ion, ?
-
20N.3.sl.TZ0.1a:
Suggest why a non-polar solvent was needed.
-
17N.3.sl.TZ0.10a:
Explain, at the molecular level, why vitamin D is soluble in fats. Use section 35 of the data booklet.
-
21M.1.sl.TZ1.10:
Which compound contains both ionic and covalent bonds?
A.
B.
C.
D.
- 21M.2.hl.TZ1.1a: Outline why metals, like iron, can conduct electricity.
- 21M.2.sl.TZ2.2b(ii): Describe metallic bonding and how it contributes to electrical conductivity.
-
18M.2.hl.TZ1.1e.ii:
Sketch two different hydrogen bonding interactions between ammonia and water.
-
18M.2.sl.TZ2.6b:
Lewis structures show electron domains and are used to predict molecular geometry.
Deduce the electron domain geometry and the molecular geometry for the NH2− ion.
- 18N.1.sl.TZ0.9: Which species has the same molecular geometry as SO32−? A. BF3 B. SO3 C. PF3 D. CO32−
- 18N.2.hl.TZ0.6d: Explain why butanoic acid is a liquid at room temperature while ethylamine is a gas at room...
- 22M.1.sl.TZ2.10: What is the type of bonding in a compound that has high boiling and melting points, poor...
-
22M.1.sl.TZ2.11:
What is the name of the compound with formula Ti3(PO4)2?
A. Titanium phosphate
B. Titanium(II) phosphate
C. Titanium(III) phosphate
D. Titanium(IV) phosphate
- 22M.2.sl.TZ2.4a(i): Outline one difference between the bonding of carbon atoms in C60 and diamond.
-
22M.2.hl.TZ2.6c(ii):
Explain the electron domain geometry of SO3.
-
19M.2.hl.TZ2.1b(i):
Deduce the Lewis (electron dot) structure of ethyne.
-
19M.2.sl.TZ1.5c(i):
Draw a Lewis (electron dot) structure of chloramine.
-
19M.2.sl.TZ1.5c(ii):
Deduce the molecular geometry of chloramine and estimate its H–N–H bond angle.
Molecular geometry:
H–N–H bond angle:
- 19M.1.sl.TZ1.10: Which species does not have resonance structures? A. C6H6 B. NH4+ C. CO32− D. O3
- 19M.1.sl.TZ2.11: Which molecule contains an incomplete octet of electrons? A. NF3 B. BF3 C. BrF D. SF2
- 19N.3.sl.TZ0.9c: Explain why stearic acid has a higher melting point than oleic acid.
- 19N.2.hl.TZ0.1b: Outline why both bonds in the ozone molecule are the same length and predict the bond length in...
Sub sections and their related questions
4.1 Ionic bonding and structure
- 16N.2.sl.TZ0.4f: Describe the structure and bonding in solid magnesium oxide.
-
16N.3.hl.TZ0.22c:
(i) Uranium hexafluoride, UF6, is used in the uranium enrichment process that produces fuel for nuclear reactors.
State the molecular shape of uranium hexafluoride.
(ii) Explain why uranium dioxide, UO2, has a very high melting point whereas uranium hexafluoride vapourises easily into gas.
- 17M.1.sl.TZ1.9: A substance has the following properties: What is the most probable structure of this...
-
17M.2.sl.TZ1.2e.i:
State the type of bonding in potassium chloride which melts at 1043 K.
-
17M.3.sl.TZ1.6b:
Lanthanum has a similar electronegativity to group 2 metals. Explain, in terms of bonding and structure, why crystalline lanthanum bromide is brittle.
-
17M.1.sl.TZ2.10:
Which bonds cause the boiling point of water to be significantly greater than that of hydrogen sulfide?
A. London (dispersion)
B. Covalent
C. Ionic
D. Hydrogen
-
17M.1.sl.TZ2.19:
Which of the following does not react with dilute HCl(aq)?
A. Na2CO3
B. Cu
C. Zn
D. CuO
-
17M.2.sl.TZ2.1c.ii:
Explain the electrical conductivity of molten Na2O and P4O10.
-
18M.2.hl.TZ1.2a:
Describe the nature of ionic bonding.
-
18M.1.sl.TZ1.9:
What is the formula of ammonium phosphate?
A. (NH3)3PO4
B. (NH4)3PO4
C. (NH4)2PO4
D. (NH3)2PO3
-
18M.2.sl.TZ1.2a:
Describe the nature of ionic bonding.
- 18M.1.sl.TZ2.9: What is the formula of magnesium nitride? A. MgN B. Mg2N3 C. Mg3N D. Mg3N2
- 18N.2.sl.TZ0.4b.ii: State a physical property of sodium oxide.
-
19M.2.hl.TZ1.3c:
Describe the structure and bonding in solid sodium oxide.
-
19M.2.hl.TZ2.5d(i):
Identify the type of bonding in sodium hydrogencarbonate.
Between sodium and hydrogencarbonate:
Between hydrogen and oxygen in hydrogencarbonate:
-
19M.1.hl.TZ1.10:
What is the IUPAC name of NiCO3?
A. nickel(II) carbonate
B. nickel carbonate
C. nickel(I) carbonate
D. nitrogen(I) carbonate
- 19M.1.hl.TZ2.9: How does a lithium atom form the most stable ion? A. The atom gains a proton to form a positive...
-
19M.2.sl.TZ1.3a(i):
Describe the structure and bonding in solid sodium oxide.
-
19M.2.sl.TZ2.5b(iii):
Identify the type of bonding in sodium hydrogencarbonate.
Between sodium and hydrogencarbonate:
Between hydrogen and oxygen in hydrogencarbonate:
- 19M.1.sl.TZ2.9: How does a lithium atom form the most stable ion? A. The atom gains a proton to form a positive...
-
19N.3.sl.TZ0.17b:
Aspirin, C6H4(OCOCH3)COOH, is only slightly soluble in water.
Outline, including an equation, how aspirin can be made more water-soluble. Use section 37 in the data booklet.
- 19N.1.sl.TZ0.9: Which is correct for all solid ionic compounds? A. High volatility B. Poor electrical...
-
20N.1.sl.TZ0.9:
Which formula is correct?
A.
B.
C.
D.
-
21M.1.sl.TZ1.10:
Which compound contains both ionic and covalent bonds?
A.
B.
C.
D.
- 21M.1.sl.TZ1.11: Which substance is most likely to be ionic?
-
21M.1.sl.TZ2.11:
What is the formula of the compound formed from Ca2+ and PO43−?
A. CaPO4
B. Ca3(PO4)2
C. Ca2(PO4)3
D. Ca(PO4)2
- 21M.1.hl.TZ2.12: Which atom has an expanded octet? A. C in CO2 B. S in SCl4 C. O in H2O2 D. P in PCl3
- 21M.2.sl.TZ1.1c(i): Describe the bonding in this type of solid.
- 21M.2.sl.TZ1.1c(iv): Suggest why chemists find it convenient to classify bonding into ionic, covalent and metallic.
- 21M.2.sl.TZ1.3a: Deduce the ratio of Fe2+:Fe3+ in Fe3O4.
- 21M.2.hl.TZ1.1d(i): Describe the bonding in this type of solid.
- 21M.2.hl.TZ1.1d(v): Suggest why chemists find it convenient to classify bonding into ionic, covalent and metallic.
- 21M.2.hl.TZ1.3a: Deduce the ratio of Fe2+:Fe3+ in Fe3O4.
- 21N.1.sl.TZ0.10: Which combination would create the strongest ionic bond?
- 21N.1.sl.TZ0.11: Which compound contains both ionic and covalent bonds? A. CH3COONa B. CH3COOH C. K2O D. ...
- 22M.1.sl.TZ1.9: A compound consists of the ions Ca2+ and PO43–. What are the name and formula of the compound?
- 22M.1.sl.TZ1.10: What is the explanation for the high melting point of sodium chloride? A. The covalent bond...
-
22M.1.sl.TZ2.9:
Which statement best describes the intramolecular bonding in HCN (l)?
A. Electrostatic attractions between H+ and CN− ions
B. Hydrogen bonding
C. Van der Waals forces and hydrogen bonding
D. Electrostatic attractions between pairs of electrons and positively charged nuclei
- 22M.1.sl.TZ2.10: What is the type of bonding in a compound that has high boiling and melting points, poor...
-
22M.1.sl.TZ2.11:
What is the name of the compound with formula Ti3(PO4)2?
A. Titanium phosphate
B. Titanium(II) phosphate
C. Titanium(III) phosphate
D. Titanium(IV) phosphate
- 22M.1.hl.TZ2.9: In which of the following compounds does ionic bonding predominate? A. HCl B. NaF C. ...
-
22M.2.sl.TZ1.1g:
State the types of bonding in magnesium, oxygen and magnesium oxide, and how the valence electrons produce these types of bonding.
-
22M.2.hl.TZ1.1g:
State the types of bonding in magnesium, oxygen and magnesium oxide, and how the valence electrons produce these types of bonding.
4.2 Covalent bonding
- 16N.1.hl.TZ0.11: How many electrons form the carbon–oxygen bond in methanal, HCHO? A. 2 B. 4 C. 8 D. 12
- 16N.3.sl.TZ0.3b: Predict the predominant type of bonding for a binary compound AB in which the electronegativity...
-
16N.3.sl.TZ0.6c:
(i) Suggest why incomplete combustion of plastic, such as polyvinyl chloride, is common in industrial and house fires.
(ii) Phthalate plasticizers such as DEHP, shown below, are frequently used in polyvinyl chloride.
With reference to bonding, suggest a reason why many adults have measurable levels of phthalates in their bodies.
- 17M.1.sl.TZ1.10: Which two atoms form the most polar bond? A. C and F B. C and Cl C. Si and F D. ...
-
17M.2.sl.TZ1.2e.ii:
A chloride of titanium, TiCl4, melts at 248 K. Suggest why the melting point is so much lower than that of KCl.
-
17M.3.sl.TZ1.6a:
Determine the type of bond present in SbBr3, showing your method. Use sections 8 and 29 of the data booklet.
- 17M.1.sl.TZ2.9: How many bonding electrons are there in the urea molecule? A. 8 B. 16 C. 20 D....
-
17M.1.sl.TZ2.10:
Which bonds cause the boiling point of water to be significantly greater than that of hydrogen sulfide?
A. London (dispersion)
B. Covalent
C. Ionic
D. Hydrogen
-
17M.2.sl.TZ2.4a.i:
State and explain the difference in bond strength between the nitrogen atoms in a hydrazine and nitrogen molecule.
- 17N.1.sl.TZ0.9: The electronegativity values of four elements are given. What is the order of increasing...
-
17N.1.sl.TZ0.10:
Which compound has the shortest C–N bond?
A. CH3NH2
B. (CH3)3CNH2
C. CH3CN
D. CH3CHNH
- 18M.1.sl.TZ2.10: Which species has the longest carbon to oxygen bond length? A. CO B. CH3OH C. ...
-
18M.1.sl.TZ2.11:
What are the predicted electron domain geometries around the carbon and both nitrogen atoms in urea, (NH2)2CO, applying VSEPR theory?

-
18M.2.sl.TZ2.6a.i:
Explain why the hydrides of group 16 elements (H2O, H2S, H2Se and H2Te) are polar molecules.
-
18M.2.sl.TZ2.6a.ii:
The graph shows the boiling points of the hydrides of group 16 elements.

Explain the increase in the boiling point from H2S to H2Te.
-
19M.2.hl.TZ2.1b(ii):
Compare, giving a reason, the length of the bond between the carbon atoms in ethyne with that in ethane, C2H6.
-
19M.2.hl.TZ2.5d(i):
Identify the type of bonding in sodium hydrogencarbonate.
Between sodium and hydrogencarbonate:
Between hydrogen and oxygen in hydrogencarbonate:
-
19M.2.sl.TZ2.1b(ii):
Compare, giving a reason, the length of the bond between the carbon atoms in ethyne with that in ethane, C2H6.
-
19M.2.sl.TZ2.5b(iii):
Identify the type of bonding in sodium hydrogencarbonate.
Between sodium and hydrogencarbonate:
Between hydrogen and oxygen in hydrogencarbonate:
- 19N.2.sl.TZ0.1b: Outline why both bonds in the ozone molecule are the same length and predict the bond length in...
- 19N.2.hl.TZ0.1c: Predict the bond angle in the ozone molecule.
- 19N.2.sl.TZ0.1d: Discuss how the different bond strengths between the oxygen atoms in O2 and O3 in the ozone layer...
-
19N.3.sl.TZ0.5b(i):
Determine the percentage of ionic bonding in alumina using sections 8 and 29 of the data booklet.
- 19N.1.sl.TZ0.10: Which compound has the shortest C to O bond? A. CH3CHO B. CO C. CO2 D. C2H5OC2H5
- 19N.2.hl.TZ0.1a: Draw the Lewis structures of oxygen, O2, and ozone, O3.
- 19N.2.hl.TZ0.1b: Outline why both bonds in the ozone molecule are the same length and predict the bond length in...
- 19N.2.hl.TZ0.1d: Discuss how the different bond strengths between the oxygen atoms in O2 and O3 in the ozone layer...
-
20N.1.hl.TZ0.9:
Which of these species contains the shortest carbon to oxygen bond length?
A.
B.
C.
D.
-
21M.1.sl.TZ1.10:
Which compound contains both ionic and covalent bonds?
A.
B.
C.
D.
-
21M.1.sl.TZ2.10:
Which compound has the shortest C to N bond?
A. HCN
B. CH3CH2NH2
C. CH3CHNH
D. (CH3)2NH
- 21M.1.sl.TZ2.12: Which is the correct order based on increasing strength? A. covalent bonds < hydrogen bonds...
- 21M.2.sl.TZ1.1c(iv): Suggest why chemists find it convenient to classify bonding into ionic, covalent and metallic.
- 21M.2.hl.TZ1.1d(v): Suggest why chemists find it convenient to classify bonding into ionic, covalent and metallic.
- 21N.1.sl.TZ0.9: Which molecule has the weakest nitrogen to nitrogen bond? A. N2 B. N2H2 C. N2H4 D.
- 21N.1.sl.TZ0.11: Which compound contains both ionic and covalent bonds? A. CH3COONa B. CH3COOH C. K2O D. ...
- 21N.2.hl.TZ0.3b(ii): Outline the reason why PCl5 is a non-polar molecule, while PCl4F is polar.
- 22M.1.sl.TZ1.10: What is the explanation for the high melting point of sodium chloride? A. The covalent bond...
- 22M.1.hl.TZ2.9: In which of the following compounds does ionic bonding predominate? A. HCl B. NaF C. ...
- 22M.2.sl.TZ2.4a(i): Outline one difference between the bonding of carbon atoms in C60 and diamond.
-
22M.2.hl.TZ2.8a(i):
Outline two differences between the bonding of carbon atoms in C60 and diamond.
-
22M.2.hl.TZ2.8a(ii):
Explain why C60 and diamond sublime at different temperatures and pressures.
4.3 Covalent structures
- 16N.1.sl.TZ0.9: Which pair of molecules has the same bond angles? A. PCl3 and BCl3 B. SO2 and CO2 C. H2O...
- 16N.1.sl.TZ0.12: Which substance has a giant covalent structure?
-
16N.2.sl.TZ0.2d:
The Lewis (electron dot) structure of the ethanedioate ion is shown below.
Outline why all the C–O bond lengths in the ethanedioate ion are the same length and suggest a value for them. Use section 10 of the data booklet.
-
16N.2.hl.TZ0.2d:
Draw the Lewis (electron dot) structure of the ethanedioate ion, –OOCCOO–.
-
16N.2.hl.TZ0.2e:
Outline why all the C–O bond lengths in the ethanedioate ion are the same length and suggest a value for them. Use section 10 of the data booklet.
-
16N.3.sl.TZ0.6c:
(i) Suggest why incomplete combustion of plastic, such as polyvinyl chloride, is common in industrial and house fires.
(ii) Phthalate plasticizers such as DEHP, shown below, are frequently used in polyvinyl chloride.
With reference to bonding, suggest a reason why many adults have measurable levels of phthalates in their bodies.
- 17M.1.sl.TZ1.9: A substance has the following properties: What is the most probable structure of this...
-
17M.1.sl.TZ1.11:
Which combination describes the sulfate(IV) ion, SO32– (also known as sulfite ion)?
- 17M.1.sl.TZ1.12: Which correctly states the strongest intermolecular forces in the compounds below?
- 17M.1.hl.TZ1.12: Which combination describes the bonding and structure in benzoic acid, C6H5COOH?
-
17M.2.sl.TZ1.4a:
Estimate the H−N−H bond angle in methanamine using VSEPR theory.
-
17M.2.hl.TZ1.5b:
State the electron domain geometry around the nitrogen atom and its hybridization in methanamine.
- 17M.1.sl.TZ2.11: What are the approximate bond angles and structure of crystalline SiO2?
-
17M.2.sl.TZ2.3b:
Deduce the Lewis (electron dot) structure and molecular geometry of PCl3.
-
17M.2.sl.TZ2.4b:
Deduce the Lewis (electron dot) structures of ozone.
-
17M.2.hl.TZ2.4b.ii:
Deduce one resonance structure of ozone and the corresponding formal charges on each oxygen atom.
-
17M.2.hl.TZ1.5a:
Estimate the H−N−H bond angle in methanamine using VSEPR theory.
-
17M.2.hl.TZ1.5c:
Ammonia reacts reversibly with water.
Explain the effect of adding ions on the position of the equilibrium. -
17N.2.sl.TZ0.3a:
Draw the Lewis (electron dot) structures of PF3 and PF4+ and use the VSEPR theory to deduce the molecular geometry of each species.
-
17N.2.sl.TZ0.3b:
Predict with a reason, whether the molecule PF3 is polar or non-polar.
-
17N.2.hl.TZ0.4a:
Draw the Lewis (electron dot) structures of PF3 and PF5 and use the VSEPR theory to deduce the molecular geometry of each species including bond angles.
-
17N.2.hl.TZ0.4b:
Predict whether the molecules PF3 and PF5 are polar or non-polar.
-
18M.2.hl.TZ1.1b:
The structural formula of urea is shown.

Predict the electron domain and molecular geometries at the nitrogen and carbon atoms, applying the VSEPR theory.

-
18M.2.hl.TZ2.7e:
Carbon and silicon are elements in group 14.
Explain why CO2 is a gas but SiO2 is a solid at room temperature.
- 18M.1.sl.TZ1.10: Which form of carbon is the poorest electrical conductor? A. Graphite B. Graphene C. ...
- 18M.1.sl.TZ1.11: What is the molecular geometry and bond angle in the molecular ion NO3−?
-
18M.2.sl.TZ1.1b:
The structural formula of urea is shown.

Predict the electron domain and molecular geometries at the nitrogen and carbon atoms, applying the VSEPR theory.

- 18M.1.sl.TZ2.10: Which species has the longest carbon to oxygen bond length? A. CO B. CH3OH C. ...
-
18M.1.sl.TZ2.11:
What are the predicted electron domain geometries around the carbon and both nitrogen atoms in urea, (NH2)2CO, applying VSEPR theory?

-
18M.2.sl.TZ2.6b:
Lewis structures show electron domains and are used to predict molecular geometry.
Deduce the electron domain geometry and the molecular geometry for the NH2− ion.
-
18M.3.sl.TZ2.1a.i:
Graphene is two-dimensional, rather than three-dimensional, material.
Justify this by using the structure of graphene and information from the table.
-
18M.3.sl.TZ2.1a.ii:
Show that graphene is over 1600 times stronger than graphite.
-
18M.3.sl.TZ2.1a.iii:
Identify a value from the table which can be used to support the information about graphene given below.

Electrons in a solid are restricted to certain ranges, or bands, of energy (vertical axis). In an insulator or semiconductor, an electron bound to an atom can break free only if it gets enough energy from heat or a passing photon to jump the “band gap”, but in graphene the gap is infinitely small.

-
18M.3.sl.TZ2.1b:
Diamond, graphene, and graphite are all network solids.
Suggest, giving a reason, the electron mobility of diamond compared to graphene.
-
18M.3.sl.TZ2.1c:
The melting point of diamond at 1 × 106 kPa is 4200 K (in the absence of oxygen).
Suggest, based on molecular structure, why graphene has a higher melting point under these conditions.
- 18N.1.sl.TZ0.9: Which species has the same molecular geometry as SO32−? A. BF3 B. SO3 C. PF3 D. CO32−
- 18N.1.sl.TZ0.10: How many lone pairs and bonding pairs of electrons surround the central chlorine atom in...
- 18N.1.sl.TZ0.12: Which molecule is polar? A. BeCl2 B. BCl3 C. NCl3 D. CCl4
-
18N.2.sl.TZ0.3b:
Draw the Lewis (electron dot) structure for BrO3− that obeys the octet rule.
- 18N.2.sl.TZ0.3c: Predict, using the VSEPR theory, the geometry of the BrO3− ion and the O−Br−O bond angles.
-
18N.2.hl.TZ0.3b.i:
Draw two Lewis (electron dot) structures for BrO3−.
- 18N.2.hl.TZ0.3c: Predict, using the VSEPR theory, the geometry of the BrO3− ion and the O−Br−O bond angles.
- 18N.2.hl.TZ0.8a: Suggest why the three-membered ring in methyloxirane is unstable.
-
19M.2.hl.TZ1.5c(i):
Draw a Lewis (electron dot) structure of chloramine.
-
19M.2.hl.TZ1.5c(iii):
Deduce the molecular geometry of chloramine and estimate its H–N–H bond angle.
Molecular geometry:
H–N–H bond angle:
-
19M.2.hl.TZ1.5c(iv):
State the type of bond formed when chloramine is protonated.
-
19M.2.hl.TZ2.1b(i):
Deduce the Lewis (electron dot) structure of ethyne.
-
19M.2.hl.TZ2.3d(ii):
State, giving a reason, the shape of the dinitrogen monoxide molecule.
-
19M.2.sl.TZ1.5c(i):
Draw a Lewis (electron dot) structure of chloramine.
-
19M.2.sl.TZ1.5c(ii):
Deduce the molecular geometry of chloramine and estimate its H–N–H bond angle.
Molecular geometry:
H–N–H bond angle:
-
19M.2.sl.TZ2.1b(i):
Deduce the Lewis (electron dot) structure of ethyne.
- 19M.1.sl.TZ1.10: Which species does not have resonance structures? A. C6H6 B. NH4+ C. CO32− D. O3
- 19M.1.sl.TZ2.11: Which molecule contains an incomplete octet of electrons? A. NF3 B. BF3 C. BrF D. SF2
- 19N.2.sl.TZ0.1a: Draw the Lewis structures of oxygen, O2, and ozone, O3.
- 19N.2.sl.TZ0.1b: Outline why both bonds in the ozone molecule are the same length and predict the bond length in...
- 19N.1.sl.TZ0.11: Which describes a resonance structure? A. Double bond can be drawn in alternative...
-
19N.1.sl.TZ0.12:
What is the structure and bonding in SiO2 (s)?
-
20N.1.sl.TZ0.10:
Which molecule is most polar?
A.
B.
C.
D.
-
20N.1.sl.TZ0.11:
Which combination correctly describes the geometry of the carbonate ion, ?
- 20N.2.sl.TZ0.2a: Predict the electron domain and molecular geometries around the oxygen atom of molecule A using...
- 20N.2.hl.TZ0.2a: Predict the electron domain and molecular geometries around the oxygen atom of molecule A using...
- 21M.1.sl.TZ1.9: The Lewis structure of methylamine is shown. What is the molecular geometry around N? A. ...
- 21M.1.sl.TZ1.12: Along which series is the bond angle increasing? A. NH3 H2O CH4 B. CH4 NH3 H2O C. H2O ...
-
21M.2.sl.TZ1.2a(i):
Draw the Lewis (electron dot) structure of hydrogen sulfide.
- 21M.2.sl.TZ1.2a(ii): Predict the shape of the hydrogen sulfide molecule.
-
21M.2.hl.TZ1.7a(i):
Draw a Lewis (electron dot) structure for ozone.
-
21M.2.sl.TZ2.2c:
Deduce the Lewis (electron dot) structure and molecular geometry of sulfur dichloride, SCl2.
-
21M.2.hl.TZ2.2c:
Deduce the Lewis (electron dot) structure and molecular geometry of sulfur tetrafluoride, SF4, and sulfur dichloride, SCl2.
-
21N.2.sl.TZ0.3a(i):
Sketch the Lewis (electron dot) structure of the P4 molecule, containing only single bonds.
- 21N.2.sl.TZ0.3b(i): Deduce the electron domain and molecular geometry using VSEPR theory, and estimate the Cl–P–Cl...
- 21N.2.sl.TZ0.3b(ii): Explain the polarity of PCl3.
-
21N.2.hl.TZ0.3a(i):
Sketch the Lewis (electron dot) structure of the P4 molecule, containing only single bonds.
- 21N.2.hl.TZ0.3b(i): Deduce the electron domain and molecular geometry using VSEPR theory, and estimate the Cl–P–Cl...
- 22M.1.sl.TZ1.11: Which molecule is most polar? A. CF4 B. CCl4 C. CHF3 D. CClF3
- 22M.1.sl.TZ1.12: For which species can resonance structures be drawn? A. HCOOH B. HCOO– C. CH3OH D. H2CO3
- 22M.1.hl.TZ1.13: What are the electron domain and molecular geometries of the XeF4 molecule?
- 22M.1.sl.TZ2.10: What is the type of bonding in a compound that has high boiling and melting points, poor...
-
22M.2.sl.TZ1.2b:
Draw the Lewis (electron dot) structure of the ammonia molecule.
-
22M.2.hl.TZ1.6a(ii):
Deduce a Lewis (electron dot) structure of the nitric acid molecule, HNO3, that obeys the octet rule, showing any non-zero formal charges on the atoms.
- 22M.2.sl.TZ2.3d(i): Draw the Lewis structure of NO3−.
- 22M.2.sl.TZ2.3d(ii): Explain the electron domain geometry of NO3−.
- 22M.2.sl.TZ2.4a(i): Outline one difference between the bonding of carbon atoms in C60 and diamond.
- 22M.2.hl.TZ2.6c(i): Draw the Lewis structure of SO3.
-
22M.2.hl.TZ2.6c(ii):
Explain the electron domain geometry of SO3.
-
22M.2.hl.TZ2.8a(i):
Outline two differences between the bonding of carbon atoms in C60 and diamond.
-
22M.2.hl.TZ2.8a(ii):
Explain why C60 and diamond sublime at different temperatures and pressures.
4.4 Intermolecular forces
- 16N.1.sl.TZ0.11: Between which pair of molecules can hydrogen bonding occur? A. CH4 and H2OB. CH3OCH3 and CF4 C....
-
16N.2.sl.TZ0.1c:
Explain why the boiling point of ethane-1,2-diol is significantly greater than that of ethene.
-
16N.3.sl.TZ0.8b:
The table below shows average figures for the percentage fatty acid composition of some common fats and oils.
(i) Deduce, with a reason, which fat or oil from the table above has the lowest iodine number.
(ii) Deduce, with a reason, which fat or oil from the table above is most likely to become rancid when exposed to the air.
(iii) The P/S index of a fat or oil is the ratio of polyunsaturated fat to saturated fat present. It is sometimes used to compare the relative health benefits of different lipids in the diet. Calculate the P/S index of beef fat and soybean oil.
(iv) Suggest why a P/S index of greater than 1 is considered beneficial to health.
(v) Cotton seed oil and corn oil have similar iodine numbers but the melting point of cotton seed oil is higher than that of corn oil. Suggest an explanation in terms of the structure and bonding in these two oils.
- 16N.3.sl.TZ0.18b: Suggest why isolation of the crude product involved the addition of ice-cold water.
- 16N.3.sl.TZ0.18c: Justify the conclusion that recrystallization increased the purity of the product, by reference...
-
16N.3.hl.TZ0.22c:
(i) Uranium hexafluoride, UF6, is used in the uranium enrichment process that produces fuel for nuclear reactors.
State the molecular shape of uranium hexafluoride.
(ii) Explain why uranium dioxide, UO2, has a very high melting point whereas uranium hexafluoride vapourises easily into gas.
- 17M.1.sl.TZ1.9: A substance has the following properties: What is the most probable structure of this...
- 17M.1.sl.TZ1.12: Which correctly states the strongest intermolecular forces in the compounds below?
- 17M.1.sl.TZ1.24: What is the order of increasing boiling point? A. C4H10 < CH3COOH < CH3CH2CHO <...
-
17M.2.sl.TZ1.2e.ii:
A chloride of titanium, TiCl4, melts at 248 K. Suggest why the melting point is so much lower than that of KCl.
-
17M.3.sl.TZ1.10b.ii:
Explain why the difference in their structures affects their melting points.
-
17M.3.sl.TZ1.11b.i:
The drain pipe of a kitchen sink can become clogged by fatty acids, such as linoleic acid, C18H32O2, but not by the trisaccharide, raffinose, C18H32O16, containing the same number of carbon atoms.
Explain why raffinose is far more water soluble than linoleic acid.
-
17M.3.sl.TZ1.13d:
The solubility of a vitamin depends on its structure.
Identify the vitamin given in section 35 of the data booklet that is the most soluble in water.
-
17M.3.sl.TZ1.19a:
Suggest why aspirin is slightly soluble in water. Refer to section 37 of the data booklet.
-
17M.1.sl.TZ2.10:
Which bonds cause the boiling point of water to be significantly greater than that of hydrogen sulfide?
A. London (dispersion)
B. Covalent
C. Ionic
D. Hydrogen
-
17M.2.sl.TZ2.4a.ii:
State why hydrazine has a higher boiling point than dinitrogen tetraoxide.
-
17M.3.sl.TZ2.8a:
Explain which one of these fatty acids has the highest boiling point.
-
17M.3.sl.TZ2.11:
Suggest, in terms of its structure, why vitamin D is fat-soluble using section 35 of the data booklet.
-
17M.3.sl.TZ2.16a:
Explain why diamorphine passes more readily than morphine through the blood-brain barrier.
- 17N.1.sl.TZ0.11: Which of the following series shows increasing hydrogen bonding with water? A. Propane <...
-
17N.2.sl.TZ0.2b:
Explain why the melting points of the group 1 metals (Li → Cs) decrease down the group.
-
17N.2.hl.TZ0.3b:
Explain why the melting points of the group 1 metals (Li → Cs) decrease down the group whereas the melting points of the group 17 elements (F → I) increase down the group.
-
17N.3.sl.TZ0.7b.i:
Describe how the structures of LDPE and HDPE affect one mechanical property of the plastics.
-
17N.3.sl.TZ0.9c:
Explain how the inclusion of carbohydrates in plastics makes them biodegradable.
-
17N.3.sl.TZ0.10a:
Explain, at the molecular level, why vitamin D is soluble in fats. Use section 35 of the data booklet.
-
17N.3.hl.TZ0.7c:
Identify the type of intermolecular bonding that is responsible for Kevlar®’s strength.
-
18M.2.hl.TZ1.1e.i:
Suggest one reason why urea is a solid and ammonia a gas at room temperature.
-
18M.2.hl.TZ1.1e.ii:
Sketch two different hydrogen bonding interactions between ammonia and water.
-
18M.2.hl.TZ2.7e:
Carbon and silicon are elements in group 14.
Explain why CO2 is a gas but SiO2 is a solid at room temperature.
-
18M.1.sl.TZ1.12:
What are the strongest intermolecular forces between molecules of propanone, CH3COCH3, in the liquid phase?
A. London (dispersion) forces
B. Covalent bonding
C. Hydrogen bonding
D. Dipole–dipole forces
-
18M.2.sl.TZ1.1e.i:
Suggest one reason why urea is a solid and ammonia a gas at room temperature.
-
18M.2.sl.TZ1.1e.ii:
Sketch two different hydrogen bonding interactions between ammonia and water.
-
18M.3.sl.TZ1.1a.i:
Part of this molecule is hydrophilic (bonds readily to water) and part hydrophobic (does not bond readily to water). Draw a circle around all of the hydrophilic part of the molecule.
-
18M.3.sl.TZ1.1a.ii:
When a small amount of palmitic acid is placed in water it disperses to form a layer on the surface that is only one molecule thick. Explain, in terms of intermolecular forces, why this occurs.
-
18M.1.sl.TZ2.12:
The compounds shown below have similar relative molecular masses. What is the correct order of increasing boiling point?
A. CH3COOH < (CH3)2CO < (CH3)2CHOH
B. CH3COOH < (CH3)2CHOH < (CH3)2CO
C. (CH3)2CO < CH3COOH < (CH3)2CHOH
D. (CH3)2CO < (CH3)2CHOH < CH3COOH
- 18N.1.sl.TZ0.11: Which compound has the highest boiling point? A. CH3CHO B. CH3CH2F C. CH3OCH3 D. ...
- 18N.2.sl.TZ0.6b: Explain why butanoic acid is a liquid at room temperature while ethylamine is a gas at room...
- 18N.2.hl.TZ0.6d: Explain why butanoic acid is a liquid at room temperature while ethylamine is a gas at room...
-
17M.3.hl.TZ1.25a:
Suggest why aspirin is slightly soluble in water. Refer to section 37 of the data booklet.
-
19M.2.hl.TZ1.2h:
Suggest how benzoic acid, Mr = 122.13, forms an apparent dimer, Mr = 244.26, when dissolved in a non-polar solvent such as hexane.
-
19M.2.hl.TZ2.1b(iii):
Identify the type of interaction that must be overcome when liquid ethyne vaporizes.
-
19M.2.hl.TZ2.1d(iii):
Explain why product B is water soluble.
-
19M.1.hl.TZ1.9:
What is the order of increasing boiling point?
A. CH3CH2CH2CH3 < CH3CH(OH)CH3 < CH3COCH3 < CH3CO2H
B. CH3CH2CH2CH3 < CH3COCH3 < CH3CH(OH)CH3 < CH3CO2H
C. CH3CO2H < CH3COCH3 < CH3CH(OH)CH3 < CH3CH2CH2CH3
D. CH3CH2CH2CH3 < CH3COCH3 < CH3CO2H < CH3CH(OH)CH3
-
19M.2.sl.TZ1.2c:
Suggest how benzoic acid, Mr = 122.13, forms an apparent dimer, Mr = 244.26, when dissolved in a non-polar solvent such as hexane.
-
19M.2.sl.TZ2.1b(iii):
Identify the type of interaction that must be overcome when liquid ethyne vaporizes.
-
19M.2.sl.TZ2.1d(iii):
Explain why product B is water soluble.
-
19M.1.sl.TZ1.9:
What is the order of increasing boiling point?
A. CH3CH2CH2CH3 < CH3CH(OH)CH3 < CH3COCH3 < CH3CO2H
B. CH3CH2CH2CH3 < CH3COCH3 < CH3CH(OH)CH3 < CH3CO2H
C. CH3CO2H < CH3COCH3 < CH3CH(OH)CH3 < CH3CH2CH2CH3
D. CH3CH2CH2CH3 < CH3COCH3 < CH3CO2H < CH3CH(OH)CH3
- 19M.1.sl.TZ1.11: Which describes an ionic compound?
- 19M.1.sl.TZ2.12: Which compound has hydrogen bonds between its molecules? A. CH4 B. CH4O C. CH3Cl D. CH2O
-
19N.2.hl.TZ0.3d(ii):
Explain why the compound C3H8O, produced in (a)(iv), has a higher boiling point than compound C3H6O, produced in d(i).
- 19N.2.sl.TZ0.6a(iv): Suggest why water vapour deviates significantly from ideal behaviour when the gases are cooled,...
- 19N.3.hl.TZ0.13b: Explain how the double-helical structure of DNA is stabilized once formed.
- 19N.3.sl.TZ0.4b: Predict, with a reason, whether isotactic or atactic polypropene has the higher melting point.
- 19N.3.sl.TZ0.9c: Explain why stearic acid has a higher melting point than oleic acid.
- 19N.3.sl.TZ0.10c: Explain why maltose, C12H22O11, is soluble in water.
-
19N.2.sl.TZ0.3d(ii):
Explain why the compound C2H6O, produced in (b), has a higher boiling point than compound C2H4O, produced in d(i).
- 19N.3.hl.TZ0.15c: Explain why maltose, C12H22O11, is soluble in water.
- 20N.2.sl.TZ0.4c: Discuss, referring to intermolecular forces present, the relative volatility of propanone and...
- 20N.2.hl.TZ0.4c: Discuss, referring to intermolecular forces present, the relative volatility of propanone and...
-
20N.3.sl.TZ0.1a:
Suggest why a non-polar solvent was needed.
- 20N.3.sl.TZ0.1c: Non-polar solvents can be toxic. Suggest a modification to the experiment which allows the...
-
20N.3.sl.TZ0.5a(ii):
Proteins are polymers of amino acids.
The mixture is composed of glycine, , and isoleucine, . Their structures can be found in section 33 of the data booklet.
Deduce, referring to relative affinities and , the identity of A1.
-
20N.3.sl.TZ0.6b(ii):
State the most significant intermolecular forces in the phospholipid in b(i).
- 20N.3.sl.TZ0.11b: Aspirin crystals are rinsed with water after recrystallization to remove impurities.Suggest why...
-
20N.3.hl.TZ0.6a(ii):
Proteins are polymers of amino acids.
The mixture is composed of glycine, , and isoleucine, . Their structures can be found in section 33 of the data booklet.
Deduce, referring to relative affinities and , the identity of A1.
- 20N.3.hl.TZ0.15b: Aspirin crystals are rinsed with water after recrystallization to remove impurities.Suggest why...
- 21M.1.sl.TZ1.24: Which series is in order of increasing boiling point? A. CH2CH2CH3OH CH3COCH3 ...
- 21M.1.sl.TZ2.9: Which compound has the greatest volatility under the same conditions? A. SO2 B. SiO2 C. ...
- 21M.1.sl.TZ2.12: Which is the correct order based on increasing strength? A. covalent bonds < hydrogen bonds...
- 21M.2.sl.TZ2.2d: Suggest, giving reasons, the relative volatilities of SCl2 and H2O.
- 21M.2.hl.TZ2.2d: Suggest, giving reasons, the relative volatilities of SCl2 and H2O.
- 21N.1.sl.TZ0.12: The following compounds have similar relative molecular masses. What is the order of increasing...
- 21N.1.sl.TZ0.13: Which alcohol is least soluble in water? A. CH3OH B. CH3CH2OH C. CH3CH2CH2OH D. ...
-
21N.2.hl.TZ0.6c(v):
Suggest a reason that the Winkler Method used to measure biochemical oxygen demand (BOD) must be done at constant temperature.
-
22M.1.sl.TZ2.9:
Which statement best describes the intramolecular bonding in HCN (l)?
A. Electrostatic attractions between H+ and CN− ions
B. Hydrogen bonding
C. Van der Waals forces and hydrogen bonding
D. Electrostatic attractions between pairs of electrons and positively charged nuclei
-
22M.1.sl.TZ2.12:
What is the main interaction between liquid CH4 molecules?
A. London (dispersion) forces
B. Dipole–dipole forces
C. Hydrogen bonding
D. Covalent bonding
- 22M.2.sl.TZ1.3c(ii): Identify the strongest force between the molecules of Compound B.
- 22M.2.hl.TZ1.5a(ii): Compound A and Compound B are both liquids at room temperature and pressure. Identify the...
-
22M.2.hl.TZ2.7c:
Suggest why hydrogen chloride, HCl, has a lower boiling point than hydrogen cyanide, HCN.
-
22M.2.hl.TZ2.8a(ii):
Explain why C60 and diamond sublime at different temperatures and pressures.
4.5 Metallic bonding
- 17M.1.sl.TZ1.9: A substance has the following properties: What is the most probable structure of this...
-
17M.2.sl.TZ1.2a:
Describe the bonding in metals.
-
17M.2.sl.TZ1.2d.ii:
Explain why an aluminium-titanium alloy is harder than pure aluminium.
-
17M.2.hl.TZ1.2d.ii:
Suggest why the melting point of vanadium is higher than that of titanium.
- 17M.1.sl.TZ2.12: Which metal has the strongest metallic bond? A. Li B. Na C. K D. Rb
-
17N.2.sl.TZ0.2b:
Explain why the melting points of the group 1 metals (Li → Cs) decrease down the group.
-
17N.2.hl.TZ0.3b:
Explain why the melting points of the group 1 metals (Li → Cs) decrease down the group whereas the melting points of the group 17 elements (F → I) increase down the group.
- 18M.1.hl.TZ1.11: Which metal has the strongest metallic bonding? A. Na B. Mg C. Al D. Ca
-
18M.2.hl.TZ1.2d.ii:
Outline why solid calcium is a good conductor of electricity.
-
18M.2.sl.TZ1.2d.ii:
Outline why solid calcium is a good conductor of electricity.
- 19M.1.hl.TZ1.11: Which combination corresponds to a strong metallic bond?
- 19M.1.hl.TZ2.10: Which combination causes the strength of metallic bonding to increase?
- 19M.1.sl.TZ1.12: Which combination corresponds to a strong metallic bond?
- 19M.1.sl.TZ2.10: Which combination causes the strength of metallic bonding to increase?
-
20N.1.sl.TZ0.12:
Which series shows the correct order of metallic bond strength from strongest to weakest?
A.
B.
C.
D.
- 20N.2.sl.TZ0.4d(iii): Describe the bonding in metals.
- 20N.2.sl.TZ0.4d(iv): Nickel alloys are used in aircraft gas turbines. Suggest a physical property altered by the...
- 20N.2.hl.TZ0.4d(v): Describe the bonding in metals.
- 20N.2.hl.TZ0.4d(vi): Nickel alloys are used in aircraft gas turbines. Suggest a physical property altered by the...
- 20N.3.sl.TZ0.4b(i): Alloying metals changes their properties. Suggest one property of magnesium that could be...
-
20N.3.hl.TZ0.4c(i):
Alloying metals changes their properties. Suggest one property of magnesium that could be improved by making a magnesium–CNT alloy.
- 21M.2.sl.TZ1.1a: Outline why metals, like iron, can conduct electricity.
- 21M.2.sl.TZ1.1c(iv): Suggest why chemists find it convenient to classify bonding into ionic, covalent and metallic.
- 21M.2.sl.TZ1.1e: Explain why the addition of small amounts of carbon to iron makes the metal harder.
- 21M.2.hl.TZ1.1a: Outline why metals, like iron, can conduct electricity.
- 21M.2.hl.TZ1.1d(v): Suggest why chemists find it convenient to classify bonding into ionic, covalent and metallic.
- 21M.2.hl.TZ1.1f: Explain why the addition of small amounts of carbon to iron makes the metal harder.
- 21M.2.sl.TZ2.2b(ii): Describe metallic bonding and how it contributes to electrical conductivity.
- 21M.2.hl.TZ2.2b(ii): Describe metallic bonding and how it contributes to electrical conductivity.
-
22M.2.hl.TZ2.5c:
Describe the bonding in iron, Fe (s).
