| Date | November 2018 | Marks available | 1 | Reference code | 18N.1.sl.TZ0.10 |
| Level | SL | Paper | 1 | Time zone | TZ0 |
| Command term | Question number | 10 | Adapted from | N/A |
Question
How many lone pairs and bonding pairs of electrons surround the central chlorine atom in ClF2+?
Markscheme
D
Examiners report
Syllabus sections
- 18M.1.sl.TZ1.10: Which form of carbon is the poorest electrical conductor? A. Graphite B. ...
- 18M.1.sl.TZ1.11: What is the molecular geometry and bond angle in the molecular ion NO3−?
-
17M.2.sl.TZ2.4b:
Deduce the Lewis (electron dot) structures of ozone.
- 22M.2.sl.TZ2.3d(i): Draw the Lewis structure of NO3−.
-
22M.2.sl.TZ1.2b:
Draw the Lewis (electron dot) structure of the ammonia molecule.
-
16N.2.sl.TZ0.2d:
The Lewis (electron dot) structure of the ethanedioate ion is shown below.
Outline why all the C–O bond lengths in the ethanedioate ion are the same length and suggest a value for them. Use section 10 of the data booklet.
- 17M.1.sl.TZ1.9: A substance has the following properties: What is the most probable structure of this...
- 22M.1.sl.TZ1.11: Which molecule is most polar? A. CF4 B. CCl4 C. CHF3 D. CClF3
- 16N.1.sl.TZ0.12: Which substance has a giant covalent structure?
- 22M.1.sl.TZ1.12: For which species can resonance structures be drawn? A. HCOOH B. HCOO– C. CH3OH D. H2CO3
-
16N.2.hl.TZ0.2d:
Draw the Lewis (electron dot) structure of the ethanedioate ion, –OOCCOO–.
- 16N.1.sl.TZ0.9: Which pair of molecules has the same bond angles? A. PCl3 and BCl3 B. SO2 and CO2 C....
-
21N.2.hl.TZ0.3a(i):
Sketch the Lewis (electron dot) structure of the P4 molecule, containing only single bonds.
- 19M.1.sl.TZ2.11: Which molecule contains an incomplete octet of electrons? A. NF3 B. BF3 C. BrF D. SF2
-
17M.2.sl.TZ2.3b:
Deduce the Lewis (electron dot) structure and molecular geometry of PCl3.
-
17M.2.hl.TZ2.4b.ii:
Deduce one resonance structure of ozone and the corresponding formal charges on each oxygen atom.
- 18M.1.sl.TZ2.10: Which species has the longest carbon to oxygen bond length? A. CO B. CH3OH C. ...
-
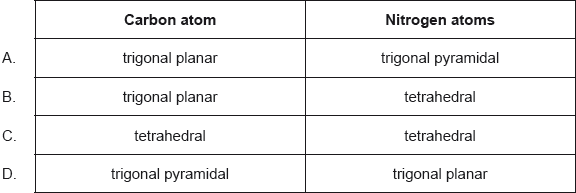
18M.1.sl.TZ2.11:
What are the predicted electron domain geometries around the carbon and both nitrogen atoms in urea, (NH2)2CO, applying VSEPR theory?
- 22M.2.hl.TZ2.6c(i): Draw the Lewis structure of SO3.
- 18N.1.sl.TZ0.12: Which molecule is polar? A. BeCl2 B. BCl3 C. NCl3 D. CCl4
- 18N.1.sl.TZ0.9: Which species has the same molecular geometry as SO32−? A. BF3 B. SO3 C. PF3 D. ...
- 18N.2.hl.TZ0.8a: Suggest why the three-membered ring in methyloxirane is unstable.
-
18N.2.hl.TZ0.3b.i:
Draw two Lewis (electron dot) structures for BrO3−.
-
18N.2.sl.TZ0.3b:
Draw the Lewis (electron dot) structure for BrO3− that obeys the octet rule.
- 18N.2.sl.TZ0.3c: Predict, using the VSEPR theory, the geometry of the BrO3− ion and the O−Br−O bond angles.
- 18N.2.hl.TZ0.3c: Predict, using the VSEPR theory, the geometry of the BrO3− ion and the O−Br−O bond angles.
- 17M.1.sl.TZ1.12: Which correctly states the strongest intermolecular forces in the compounds below?
-
17M.1.sl.TZ1.11:
Which combination describes the sulfate(IV) ion, SO32– (also known as sulfite ion)?
-
17M.2.hl.TZ1.5c:
Ammonia reacts reversibly with water.
Explain the effect of adding ions on the position of the equilibrium. - 17M.1.hl.TZ1.12: Which combination describes the bonding and structure in benzoic acid, C6H5COOH?
-
18M.3.sl.TZ2.1c:
The melting point of diamond at 1 × 106 kPa is 4200 K (in the absence of oxygen).
Suggest, based on molecular structure, why graphene has a higher melting point under these conditions.
-
18M.2.sl.TZ2.6b:
Lewis structures show electron domains and are used to predict molecular geometry.
Deduce the electron domain geometry and the molecular geometry for the NH2− ion.
-
18M.3.sl.TZ2.1a.ii:
Show that graphene is over 1600 times stronger than graphite.
-
18M.2.hl.TZ1.1b:
The structural formula of urea is shown.
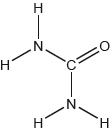
Predict the electron domain and molecular geometries at the nitrogen and carbon atoms, applying the VSEPR theory.
-
19M.2.hl.TZ1.5c(iv):
State the type of bond formed when chloramine is protonated.
-
19M.2.sl.TZ1.5c(i):
Draw a Lewis (electron dot) structure of chloramine.
- 19M.1.sl.TZ1.10: Which species does not have resonance structures? A. C6H6 B. NH4+ C. CO32− D. O3
-
17N.2.sl.TZ0.3b:
Predict with a reason, whether the molecule PF3 is polar or non-polar.
- 22M.1.hl.TZ1.13: What are the electron domain and molecular geometries of the XeF4 molecule?
- 17M.1.sl.TZ2.11: What are the approximate bond angles and structure of crystalline SiO2?
-
17M.2.hl.TZ1.5a:
Estimate the H−N−H bond angle in methanamine using VSEPR theory.
- 19N.2.sl.TZ0.1a: Draw the Lewis structures of oxygen, O2, and ozone, O3.
- 19N.2.sl.TZ0.1b: Outline why both bonds in the ozone molecule are the same length and predict the bond length...
-
16N.2.hl.TZ0.2e:
Outline why all the C–O bond lengths in the ethanedioate ion are the same length and suggest a value for them. Use section 10 of the data booklet.
-
21M.2.hl.TZ1.7a(i):
Draw a Lewis (electron dot) structure for ozone.
-
19M.2.sl.TZ2.1b(i):
Deduce the Lewis (electron dot) structure of ethyne.
-
17M.2.sl.TZ1.4a:
Estimate the H−N−H bond angle in methanamine using VSEPR theory.
-
17N.2.sl.TZ0.3a:
Draw the Lewis (electron dot) structures of PF3 and PF4+ and use the VSEPR theory to deduce the molecular geometry of each species.
-
17M.2.hl.TZ1.5b:
State the electron domain geometry around the nitrogen atom and its hybridization in methanamine.
-
19N.1.sl.TZ0.12:
What is the structure and bonding in SiO2 (s)?
- 19N.1.sl.TZ0.11: Which describes a resonance structure? A. Double bond can be drawn in alternative...
-
18M.2.hl.TZ2.7e:
Carbon and silicon are elements in group 14.
Explain why CO2 is a gas but SiO2 is a solid at room temperature.
-
19M.2.hl.TZ1.5c(i):
Draw a Lewis (electron dot) structure of chloramine.
-
16N.3.sl.TZ0.6c:
(i) Suggest why incomplete combustion of plastic, such as polyvinyl chloride, is common in industrial and house fires.
(ii) Phthalate plasticizers such as DEHP, shown below, are frequently used in polyvinyl chloride.
With reference to bonding, suggest a reason why many adults have measurable levels of phthalates in their bodies.
-
17N.2.hl.TZ0.4a:
Draw the Lewis (electron dot) structures of PF3 and PF5 and use the VSEPR theory to deduce the molecular geometry of each species including bond angles.
-
17N.2.hl.TZ0.4b:
Predict whether the molecules PF3 and PF5 are polar or non-polar.
-
18M.3.sl.TZ2.1a.iii:
Identify a value from the table which can be used to support the information about graphene given below.
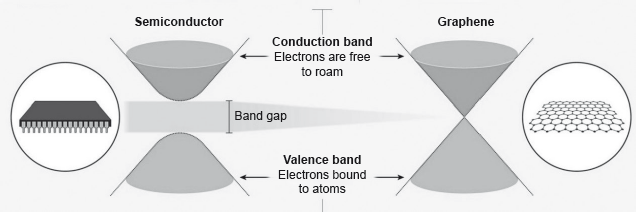
Electrons in a solid are restricted to certain ranges, or bands, of energy (vertical axis). In an insulator or semiconductor, an electron bound to an atom can break free only if it gets enough energy from heat or a passing photon to jump the “band gap”, but in graphene the gap is infinitely small.

-
18M.3.sl.TZ2.1b:
Diamond, graphene, and graphite are all network solids.
Suggest, giving a reason, the electron mobility of diamond compared to graphene.
-
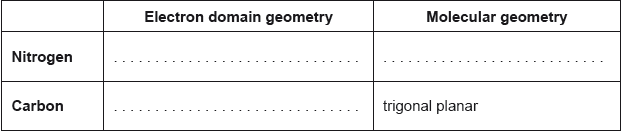
18M.2.sl.TZ1.1b:
The structural formula of urea is shown.
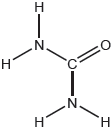
Predict the electron domain and molecular geometries at the nitrogen and carbon atoms, applying the VSEPR theory.
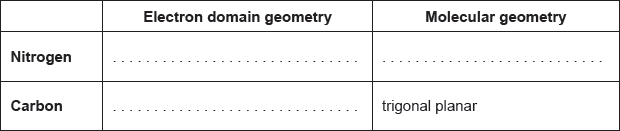
- 21M.1.sl.TZ1.9: The Lewis structure of methylamine is shown. What is the molecular geometry around N? A. ...
- 21M.1.sl.TZ1.12: Along which series is the bond angle increasing? A. NH3 H2O CH4 B. CH4 NH3 H2O C. ...
-
19M.2.sl.TZ1.5c(ii):
Deduce the molecular geometry of chloramine and estimate its H–N–H bond angle.
Molecular geometry:
H–N–H bond angle:
-
21N.2.sl.TZ0.3a(i):
Sketch the Lewis (electron dot) structure of the P4 molecule, containing only single bonds.
-
18M.3.sl.TZ2.1a.i:
Graphene is two-dimensional, rather than three-dimensional, material.
Justify this by using the structure of graphene and information from the table.
- 21N.2.sl.TZ0.3b(i): Deduce the electron domain and molecular geometry using VSEPR theory, and estimate the...
-
21M.2.sl.TZ1.2a(i):
Draw the Lewis (electron dot) structure of hydrogen sulfide.
- 21M.2.sl.TZ1.2a(ii): Predict the shape of the hydrogen sulfide molecule.
-
21M.2.sl.TZ2.2c:
Deduce the Lewis (electron dot) structure and molecular geometry of sulfur dichloride, SCl2.
-
22M.2.hl.TZ2.6c(ii):
Explain the electron domain geometry of SO3.
-
20N.1.sl.TZ0.11:
Which combination correctly describes the geometry of the carbonate ion, ?
-
19M.2.hl.TZ2.1b(i):
Deduce the Lewis (electron dot) structure of ethyne.
-
21M.2.hl.TZ2.2c:
Deduce the Lewis (electron dot) structure and molecular geometry of sulfur tetrafluoride, SF4, and sulfur dichloride, SCl2.
-
19M.2.hl.TZ2.3d(ii):
State, giving a reason, the shape of the dinitrogen monoxide molecule.
- 20N.2.sl.TZ0.2a: Predict the electron domain and molecular geometries around the oxygen atom of molecule A...
- 20N.2.hl.TZ0.2a: Predict the electron domain and molecular geometries around the oxygen atom of molecule A...
-
22M.2.hl.TZ2.8a(i):
Outline two differences between the bonding of carbon atoms in C60 and diamond.
-
22M.2.hl.TZ2.8a(ii):
Explain why C60 and diamond sublime at different temperatures and pressures.
- 22M.2.sl.TZ2.3d(ii): Explain the electron domain geometry of NO3−.
- 22M.2.sl.TZ2.4a(i): Outline one difference between the bonding of carbon atoms in C60 and diamond.
-
19M.2.hl.TZ1.5c(iii):
Deduce the molecular geometry of chloramine and estimate its H–N–H bond angle.
Molecular geometry:
H–N–H bond angle:
-
20N.1.sl.TZ0.10:
Which molecule is most polar?
A.
B.
C.
D.
-
22M.2.hl.TZ1.6a(ii):
Deduce a Lewis (electron dot) structure of the nitric acid molecule, HNO3, that obeys the octet rule, showing any non-zero formal charges on the atoms.
- 22M.1.sl.TZ2.10: What is the type of bonding in a compound that has high boiling and melting points, poor...
- 21N.2.hl.TZ0.3b(i): Deduce the electron domain and molecular geometry using VSEPR theory, and estimate the...
- 21N.2.sl.TZ0.3b(ii): Explain the polarity of PCl3.

