| Date | May 2022 | Marks available | 1 | Reference code | 22M.2.hl.TZ2.6 |
| Level | HL | Paper | 2 | Time zone | TZ2 |
| Command term | Draw | Question number | 6 | Adapted from | N/A |
Question
Sulfur trioxide is produced from sulfur dioxide.
2SO2 (g) + O2 (g) 2SO3 (g) ΔH = −196 kJ mol−1
The reaction between sulfur dioxide and oxygen can be carried out at different temperatures.
Outline, giving a reason, the effect of a catalyst on a reaction.
On the axes, sketch Maxwell–Boltzmann energy distribution curves for the reacting species at two temperatures T1 and T2, where T2 > T1.
Explain the effect of increasing temperature on the yield of SO3.
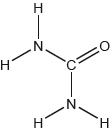
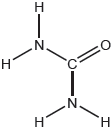
Draw the Lewis structure of SO3.
Explain the electron domain geometry of SO3.
State the product formed from the reaction of SO3 with water.
State the meaning of a strong Brønsted–Lowry acid.
Markscheme
increases rate AND lower Ea ✔
provides alternative pathway «with lower Ea»
OR
more/larger fraction of molecules have the «lower» Ea ✔
Accept description of how catalyst lowers Ea for M2 (e.g. “reactants adsorb on surface «of catalyst»”, “reactant bonds weaken «when adsorbed»”, “helps favorable orientation of molecules”).
both axes correctly labelled ✔
peak of T2 curve lower AND to the right of T1 curve ✔
lines begin at origin AND correct shape of curves AND T2 must finish above T1 ✔
Accept “probability «density» / number of particles / N / fraction” on y-axis.
Accept “kinetic E/KE/Ek” but not just “Energy/E” on x-axis.
decrease AND equilibrium shifts left / favours reverse reaction ✔
«forward reaction is» exothermic / ΔH is negative ✔
✔
Note:
Accept any of the above structures as formal charge is not being assessed.
three electron domains «attached to the central atom» ✔
repel/as far away as possible /120° «apart» ✔
sulfuric acid/H2SO4 ✔
Accept “disulfuric acid/H2S2O7”.
fully ionizes/dissociates ✔
proton/H+ «donor »✔
Examiners report
Overall well answered though some answers were directed to explain the specific example rather than the simple and standard definition of the effect of a catalyst.
Few got the 3 marks for this standard question (average mark 1.7), the most common error being incomplete/incorrect labelling of axes, curves beginning above 0 on y-axis or inverted curves.
Many candidates got one mark at least, sometimes failing to state the effect on the production of SO3 though they knew this quite obviously. This failure to read the question properly also resulted in an incorrect prediction based exclusively on kinetics instead of using the information provided to guide their answers.
Drawing the Lewis structure of SO3 proved to be challenging, with lots of incorrect shapes, lone pair on S, etc.; accepting all resonant structures allowed many candidates to get the mark which was fair considering no formal charge estimation was required.
Most were focussed on the shape itself instead of explaining what led them to suggest that shape; number of electron domains allowed most candidates to get one mark and eventually a mention of bond angles resulted in only 35% getting both marks. In general, students were not able to provide clear explanations for the shape (not a language issue) but rather were happy to state the molecular geometry which they knew, but wasn't what was actually required for the mark.
6(d)(i)-(ii): These simple questions could be expected to be answered by all HL candidates. However 20% of the candidates suggested hydroxides or hydrogen as products of an aqueous dissolution of sulphur oxide. In the case of the definition of a strong Brønsted-Lowry acid, only 50% got both marks, often failing to define "strong" but in other cases defining them as bases even.