| Date | May 2021 | Marks available | 4 | Reference code | 21M.2.hl.TZ2.2 |
| Level | HL | Paper | 2 | Time zone | TZ2 |
| Command term | Deduce | Question number | 2 | Adapted from | N/A |
Question
The properties of elements can be predicted from their position in the periodic table.
Explain why Si has a smaller atomic radius than Al.
Explain why the first ionization energy of sulfur is lower than that of phosphorus.
State the condensed electron configurations for Cr and Cr3+.
Describe metallic bonding and how it contributes to electrical conductivity.
Deduce, giving a reason, which complex ion [Cr(CN)6]3− or [Cr(OH)6]3− absorbs higher energy light. Use section 15 of the data booklet.
[Cr(OH)6]3− forms a green solution. Estimate a wavelength of light absorbed by this complex, using section 17 of the data booklet.
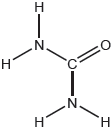
Deduce the Lewis (electron dot) structure and molecular geometry of sulfur tetrafluoride, SF4, and sulfur dichloride, SCl2.
Suggest, giving reasons, the relative volatilities of SCl2 and H2O.
Markscheme
nuclear charge/number of protons/Z/Zeff increases «causing a stronger pull on the outer electrons» ✓
same number of shells/«outer» energy level/shielding ✓
P has «three» unpaired electrons in 3p sub-level AND S has one full 3p orbital «and two 3p orbitals with unpaired electrons»
OR
P: [Ne]3s23px13py13pz1 AND S: [Ne]3s23px23py13pz1 ✓
Accept orbital diagrams for 3p sub-level for M1. Ignore other orbitals or sub-levels.
repulsion between paired electrons in sulfur «and therefore easier to remove» ✓
Accept “removing electron from S gives more stable half-filled sub-level" for M2.
Cr:
[Ar] 4s13d5 ✓
Cr3+:
[Ar] 3d3 ✓
Accept “[Ar] 3d54s1”.
Accept “[Ar] 3d34s0”.
Award [1 max] for two correct full electron configurations “1s22s22p63s23p64s13d5 AND 1s22s22p63s23p63d3”.
Award [1 max] for 4s13d5 AND 3d3.
electrostatic attraction ✓
between «a lattice of» cations/positive «metal» ions AND «a sea of» delocalized electrons ✓
mobile electrons responsible for conductivity
OR
electrons move when a voltage/potential difference/electric field is applied ✓
Do not accept “nuclei” for “cations/positive ions” in M2.
Accept “mobile/free” for “delocalized” electrons in M2.
Accept “electrons move when connected to a cell/battery/power supply” OR “electrons move when connected in a circuit” for M3.
[Cr(CN)6]3− AND CN−/ligand causes larger splitting «in d-orbitals compared to OH−»
OR
[Cr(CN)6]3− AND CN−/ligand associated with a higher Δ/«crystal field» splitting energy/energy difference «in the spectrochemical series compared to OH− » ✓
Accept “[Cr(CN)6]3− AND «CN−» strong field ligand”.
any value or range between 647 and 700 nm ✓
SF4/SCl2 structure does not have to be 3-D for mark.
Penalize missing lone pairs of electrons on halogens once only.
Accept any combination of dots, lines or crosses for bonds/lone pairs.
Accept “non-linear” for SCl2 molecular geometry.
Award [1] for two correct electron domain geometries, e.g. trigonal bipyramidal for SF4 and tetrahedral for SCl2.
H2O forms hydrogen bonding «while SCl2 does not» ✓
SCl2 «much» stronger London/dispersion/«instantaneous» induced dipole-induced dipole forces ✓
Alternative 1:
H2O less volatile AND hydrogen bonding stronger «than dipole–dipole and dispersion forces» ✓
Alternative 2:
SCl2 less volatile AND effect of dispersion forces «could be» greater than hydrogen bonding ✓
Ignore reference to Van der Waals.
Accept “SCl2 has «much» larger molar mass/electron density” for M2.