| Date | November 2019 | Marks available | 1 | Reference code | 19N.1.sl.TZ0.10 |
| Level | SL | Paper | 1 | Time zone | TZ0 |
| Command term | Deduce | Question number | 10 | Adapted from | N/A |
Question
Which compound has the shortest C to O bond?
A. CH3CHO
B. CO
C. CO2
D. C2H5OC2H5
Markscheme
B
Examiners report
Syllabus sections
-
17N.1.sl.TZ0.10:
Which compound has the shortest C–N bond?
A. CH3NH2
B. (CH3)3CNH2
C. CH3CN
D. CH3CHNH
- 17M.1.sl.TZ2.9: How many bonding electrons are there in the urea molecule? A. 8 B. 16 C. ...
- 17M.1.sl.TZ1.10: Which two atoms form the most polar bond? A. C and F B. C and Cl C. Si and...
-
17M.1.sl.TZ2.10:
Which bonds cause the boiling point of water to be significantly greater than that of hydrogen sulfide?
A. London (dispersion)
B. Covalent
C. Ionic
D. Hydrogen
- 22M.1.sl.TZ1.10: What is the explanation for the high melting point of sodium chloride? A. The covalent bond...
- 22M.1.hl.TZ2.9: In which of the following compounds does ionic bonding predominate? A. HCl B. NaF C. ...
- 19N.2.hl.TZ0.1a: Draw the Lewis structures of oxygen, O2, and ozone, O3.
-
18M.2.sl.TZ2.6a.ii:
The graph shows the boiling points of the hydrides of group 16 elements.
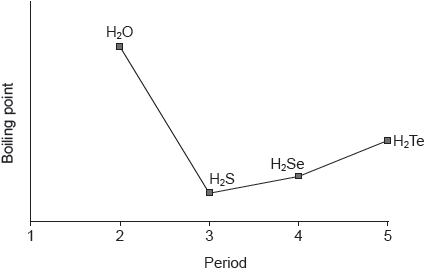
Explain the increase in the boiling point from H2S to H2Te.
- 18M.1.sl.TZ2.10: Which species has the longest carbon to oxygen bond length? A. CO B. CH3OH C. ...
-
18M.1.sl.TZ2.11:
What are the predicted electron domain geometries around the carbon and both nitrogen atoms in urea, (NH2)2CO, applying VSEPR theory?
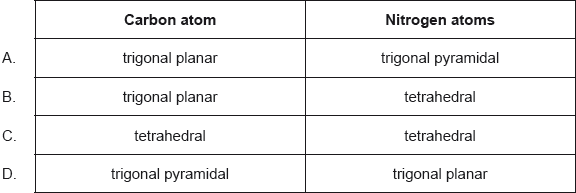
- 21N.1.sl.TZ0.9: Which molecule has the weakest nitrogen to nitrogen bond? A. N2 B. N2H2 C. N2H4 D.
- 21N.1.sl.TZ0.11: Which compound contains both ionic and covalent bonds? A. CH3COONa B. CH3COOH C. ...
- 17N.1.sl.TZ0.9: The electronegativity values of four elements are given. What is the order of increasing...
-
19M.2.hl.TZ2.1b(ii):
Compare, giving a reason, the length of the bond between the carbon atoms in ethyne with that in ethane, C2H6.
- 16N.3.sl.TZ0.3b: Predict the predominant type of bonding for a binary compound AB in which the...
- 16N.1.hl.TZ0.11: How many electrons form the carbon–oxygen bond in methanal, HCHO? A. 2 B. 4 C. 8 D. 12
- 19N.2.hl.TZ0.1b: Outline why both bonds in the ozone molecule are the same length and predict the bond length...
- 19N.2.hl.TZ0.1d: Discuss how the different bond strengths between the oxygen atoms in O2 and O3 in the ozone...
- 19N.2.sl.TZ0.1b: Outline why both bonds in the ozone molecule are the same length and predict the bond length...
- 19N.2.hl.TZ0.1c: Predict the bond angle in the ozone molecule.
-
17M.3.sl.TZ1.6a:
Determine the type of bond present in SbBr3, showing your method. Use sections 8 and 29 of the data booklet.
-
19N.3.sl.TZ0.5b(i):
Determine the percentage of ionic bonding in alumina using sections 8 and 29 of the data booklet.
-
19M.2.sl.TZ2.1b(ii):
Compare, giving a reason, the length of the bond between the carbon atoms in ethyne with that in ethane, C2H6.
-
19M.2.sl.TZ2.5b(iii):
Identify the type of bonding in sodium hydrogencarbonate.
Between sodium and hydrogencarbonate:
Between hydrogen and oxygen in hydrogencarbonate:
- 19N.2.sl.TZ0.1d: Discuss how the different bond strengths between the oxygen atoms in O2 and O3 in the ozone...
-
17M.2.sl.TZ1.2e.ii:
A chloride of titanium, TiCl4, melts at 248 K. Suggest why the melting point is so much lower than that of KCl.
-
17M.2.sl.TZ2.4a.i:
State and explain the difference in bond strength between the nitrogen atoms in a hydrazine and nitrogen molecule.
-
16N.3.sl.TZ0.6c:
(i) Suggest why incomplete combustion of plastic, such as polyvinyl chloride, is common in industrial and house fires.
(ii) Phthalate plasticizers such as DEHP, shown below, are frequently used in polyvinyl chloride.
With reference to bonding, suggest a reason why many adults have measurable levels of phthalates in their bodies.
-
19M.2.hl.TZ2.5d(i):
Identify the type of bonding in sodium hydrogencarbonate.
Between sodium and hydrogencarbonate:
Between hydrogen and oxygen in hydrogencarbonate:
-
21M.1.sl.TZ1.10:
Which compound contains both ionic and covalent bonds?
A.
B.
C.
D.
- 21M.2.hl.TZ1.1d(v): Suggest why chemists find it convenient to classify bonding into ionic, covalent and metallic.
- 21M.1.sl.TZ2.12: Which is the correct order based on increasing strength? A. covalent bonds < hydrogen...
-
21M.1.sl.TZ2.10:
Which compound has the shortest C to N bond?
A. HCN
B. CH3CH2NH2
C. CH3CHNH
D. (CH3)2NH
-
20N.1.hl.TZ0.9:
Which of these species contains the shortest carbon to oxygen bond length?
A.
B.
C.
D.
-
22M.2.hl.TZ2.8a(i):
Outline two differences between the bonding of carbon atoms in C60 and diamond.
-
22M.2.hl.TZ2.8a(ii):
Explain why C60 and diamond sublime at different temperatures and pressures.
- 22M.2.sl.TZ2.4a(i): Outline one difference between the bonding of carbon atoms in C60 and diamond.
-
18M.2.sl.TZ2.6a.i:
Explain why the hydrides of group 16 elements (H2O, H2S, H2Se and H2Te) are polar molecules.
- 21M.2.sl.TZ1.1c(iv): Suggest why chemists find it convenient to classify bonding into ionic, covalent and metallic.
- 21N.2.hl.TZ0.3b(ii): Outline the reason why PCl5 is a non-polar molecule, while PCl4F is polar.

