| Date | November 2019 | Marks available | 2 | Reference code | 19N.2.sl.TZ0.1 |
| Level | SL | Paper | 2 | Time zone | TZ0 |
| Command term | Draw | Question number | 1 | Adapted from | N/A |
Question
The equations show steps in the formation and decomposition of ozone in the stratosphere, some of which absorb ultraviolet light.
Step 1 O2 → 2O•
Step 2 O• + O2 → O3
Step 3 O3 → O• + O2
Step 4 O• + O3 → 2O2
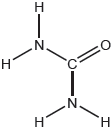
Draw the Lewis structures of oxygen, O2, and ozone, O3.
Outline why both bonds in the ozone molecule are the same length and predict the bond length in the ozone molecule. Refer to section 10 of the data booklet.
Reason:
Length:
Distinguish ultraviolet light from visible light in terms of wavelength and energy.
Discuss how the different bond strengths between the oxygen atoms in O2 and O3 in the ozone layer affect radiation reaching the Earth’s surface.
Markscheme
NOTES: Coordinate bond may be represented by an arrow.
Do not accept delocalized structure for ozone.
resonance «structures»
OR
delocalization of «the double/pi bond» electrons ✔
121 «pm» < length < 148 «pm» ✔
NOTE: Accept any length between these two values.
«UV» shorter wavelength AND higher energy «than visible» ✔
«bond» in O2 stronger than in O3 ✔
ozone absorbs lower frequency/energy «radiation than oxygen»
OR
ozone absorbs longer wavelength «radiation than oxygen» ✔
NOTE: Accept ozone «layer» absorbs a range of frequencies.