| Date | May 2022 | Marks available | 2 | Reference code | 22M.2.sl.TZ2.3 |
| Level | SL | Paper | 2 | Time zone | TZ2 |
| Command term | Explain | Question number | 3 | Adapted from | N/A |
Question
Sulfur trioxide is produced from sulfur dioxide.
2SO2 (g) + O2 (g) 2SO3 (g) ΔH = −196 kJ mol−1
The reaction between sulfur dioxide and oxygen can be carried out at different temperatures.
Nitric acid, HNO3, is another strong Brønsted–Lowry acid. Its conjugate base is the nitrate ion, NO3−
Outline, giving a reason, the effect of a catalyst on a reaction.
On the axes, sketch Maxwell–Boltzmann energy distribution curves for the reacting species at two temperatures T1 and T2, where T2 > T1.
Explain the effect of increasing temperature on the yield of SO3.
State the product formed from the reaction of SO3 with water.
State the meaning of a strong Brønsted–Lowry acid.
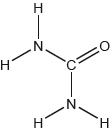
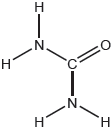
Draw the Lewis structure of NO3−.
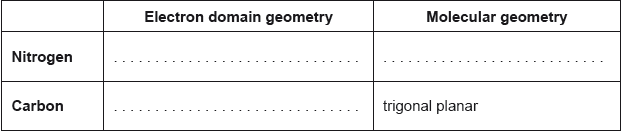
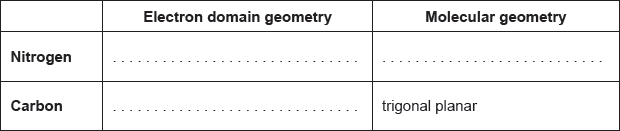
Explain the electron domain geometry of NO3−.
Markscheme
increases rate AND lower Ea ✔
provides alternative pathway «with lower Ea»
OR
more/larger fraction of molecules have the «lower» Ea ✔
Accept description of how catalyst lowers Ea for M2 (e.g. “reactants adsorb on surface «of catalyst»”, “reactant bonds weaken «when adsorbed»”, “helps favorable orientation of molecules”).
both axes correctly labelled ✔
peak of T2 curve lower AND to the right of T1 curve ✔
lines begin at origin AND correct shape of curves AND T2 must finish above T1 ✔
Accept “probability «density» / number of particles / N / fraction” on y-axis.
Accept “kinetic E/KE/Ek” but not just “Energy/E” on x-axis.
decrease AND equilibrium shifts left / favours reverse reaction ✔
«forward reaction is» exothermic / ΔH is negative ✔
sulfuric acid/H2SO4 ✔
Accept “disulfuric acid/H2S2O7”.
fully ionizes/dissociates ✔
proton/H+ «donor » ✔
Do not accept the delocalised structure.
Accept any combination of dots, crosses and lines.
Coordinate/dative bond may be represented by an arrow.
three electron domains repel
OR
three electron domains as far away as possible ✔
trigonal planar
OR
«all» angles are 120° ✔
Examiners report
A generally well-answered question. Most candidates explained the effect of a catalyst on a reaction correctly. A small proportion of candidates thought the catalyst increased the frequency of collisions. Some candidates focussed on the effect of the catalyst on an equilibrium since the equation above the question was that of a reversible reaction. These candidates usually still managed to gain at least the first marking point by stating that both forward and reverse reaction rates were increased due to the lower activation energy. Most candidates mentioned the alternative pathway for the second mark, and some gave a good discussion about the increase in the number of molecules or collisions with E≥Ea. A few candidates lost one of the marks for not explicitly stating the effect of a catalyst (that it increases the rate of the reaction).
The average mark scored for the Maxwell-Boltzmann distribution curves sketch was 1.5 out of 3 marks and the question had a strong correlation with the candidates who did well overall. The majority of candidates were familiar with the shapes of the curves. The most commonly lost mark was missing or incorrect labels on the axes. Sometimes candidates added the labels but did not specify “kinetic” energy for the x-axis. As for the curves, some candidates reversed the labels T1 and T2, some made the two curves meet at high energy or even cross, and some did not have the correct relationship between the peaks of T1 and T2.
Another question that showed a strong correlation with the candidates who did well overall. The average mark was 1 out of 2 marks. Many candidates explained the effect of an increase in temperature on the yield of SO3 correctly and thoroughly. One of the common mistakes was to miss the fact that it was an equilibrium and reason that yield would not change due to an increase in the rate of reaction. Unfortunately, a number of candidates also deduced that yield would increase due to the increase in rate. Other candidates recognized that it was an exothermic reaction but deduced the equilibrium would shift to the right giving a higher yield of SO3.
A very well answered question. 70% of the candidates stated H2SO4 as the product from the reaction of SO3 with water.
While a straightforward question, many candidates only answered part of the question - either focussing on the “strong” or on the “Brønsted-Lowry acid”. The average mark on this question was 1.2 out of 2 marks.
Only 20% of the candidates scored the mark for the Lewis structure of NO3-. Mistakes included: missing charge, missing lone pairs, 3 single bonds, 2 double bonds.
The majority of candidates deduced the correct electron domain geometry scoring the first mark including cases of ECF. Only a small number of candidates satisfied the requirements of the markscheme for the explanation.