| Date | May 2017 | Marks available | 2 | Reference code | 17M.2.sl.TZ2.4 |
| Level | SL | Paper | 2 | Time zone | TZ2 |
| Command term | Deduce | Question number | 4 | Adapted from | N/A |
Question
Bonds can be formed in many ways.
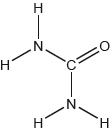
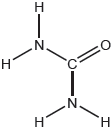
The landing module for the Apollo mission used rocket fuel made from a mixture of hydrazine, N2H4, and dinitrogen tetraoxide, N2O4.
N2H4(l) + N2O4(l) → 3N2(g) + 4H2O(g)
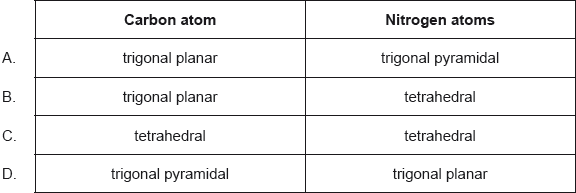
State and explain the difference in bond strength between the nitrogen atoms in a hydrazine and nitrogen molecule.
State why hydrazine has a higher boiling point than dinitrogen tetraoxide.
Determine the oxidation state of nitrogen in the two reactants.
Deduce, giving a reason, which species is the reducing agent.
Deduce the Lewis (electron dot) structures of ozone.
Markscheme
triple bond in nitrogen «molecule» AND single bond in hydrazine
triple bond stronger than single bond
OR
more shared «pairs of» electrons make bond stronger/attract nuclei more
Accept bond enthalpy values from data booklet (158 and 945 kJmol–1).
[2 marks]
hydrogen bonding «between molecules, dinitrogen tetraoxide does not»
[1 mark]
N2H4: –2 AND N2O4: +4
[1 mark]
N2H4 AND oxidized/oxidation state increases
OR
N2H4 AND loses hydrogen
OR
N2H4 AND reduces/removes oxygen from N2O4
Accept “N2H4 AND gives electrons «to N2O4»”.
[1 mark]
Accept any combination of lines, dots or crosses to represent electrons.
Do not penalize missing lone pairs if already done in 3b.
Do not accept structure that represents 1.5 bonds.
[2 marks]