| Date | May 2017 | Marks available | 2 | Reference code | 17M.2.sl.TZ2.3 |
| Level | SL | Paper | 2 | Time zone | TZ2 |
| Command term | Deduce | Question number | 3 | Adapted from | N/A |
Question
PCl5(g) and Cl2(g) were placed in a sealed flask and allowed to reach equilibrium at 200 °C. The enthalpy change, ΔH, for the decomposition of PCl5(g) is positive.
Deduce the equilibrium constant expression, Kc, for the decomposition of PCl5(g).
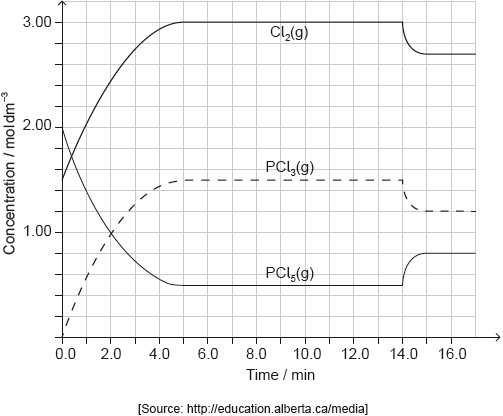
Deduce, giving a reason, the factor responsible for establishing the new equilibrium after 14 minutes.
Deduce the Lewis (electron dot) structure and molecular geometry of PCl3.
Markscheme
«Kc» =
[1 mark]
decrease in temperature
endothermic «reaction» AND «equilibrium» shifts to the left/reactants
OR
endothermic «reaction» AND Kc decreases
OR
endothermic «reaction» AND concentration of PCl5 increased/concentration of PCl3 and Cl2 decreased
OR
«equilibrium» shifts in exothermic direction
Do not accept “temperature change”.
Accept “ΔH positive” in place of “endothermic”.
Accept “products” instead of “PCl3 and Cl2”.
[2 marks]
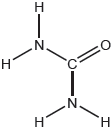
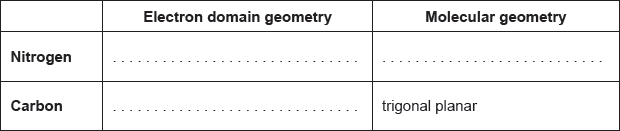
Lewis structure:
Molecular geometry:
trigonal/triangular pyramidal
Penalize missing lone pairs once only between this question and 4(b).
Accept any combination of lines, dots or crosses to represent electrons.
Do not apply ECF.
[2 marks]