| Date | May 2021 | Marks available | 1 | Reference code | 21M.1.sl.TZ2.12 |
| Level | SL | Paper | 1 | Time zone | TZ2 |
| Command term | Deduce | Question number | 12 | Adapted from | N/A |
Question
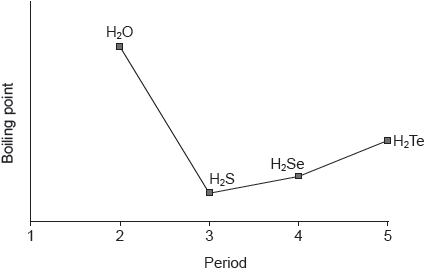
Which is the correct order based on increasing strength?
A. covalent bonds < hydrogen bonds < dipole–dipole forces < dispersion forces
B. dipole–dipole forces < dispersion forces < hydrogen bonds < covalent bonds
C. dispersion forces < dipole–dipole forces < hydrogen bonds < covalent bonds
D. dispersion forces < dipole–dipole forces < covalent bonds < hydrogen bonds
Markscheme
C
Examiners report
[N/A]