| Date | May 2019 | Marks available | 2 | Reference code | 19M.2.sl.TZ1.5 |
| Level | SL | Paper | 2 | Time zone | TZ1 |
| Command term | Deduce | Question number | 5 | Adapted from | N/A |
Question
Both vinegar (a dilute aqueous solution of ethanoic acid) and bleach are used as cleaning agents.
Bleach reacts with ammonia, also used as a cleaning agent, to produce the poisonous compound chloramine, NH2Cl.
Outline why ethanoic acid is classified as a weak acid.
A solution of bleach can be made by reacting chlorine gas with a sodium hydroxide solution.
Cl2 (g) + 2NaOH (aq) NaOCl (aq) + NaCl (aq) + H2O (l)
Suggest, with reference to Le Châtelier’s principle, why it is dangerous to mix vinegar and bleach together as cleaners.
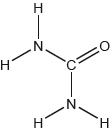
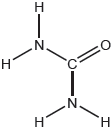
Draw a Lewis (electron dot) structure of chloramine.
Deduce the molecular geometry of chloramine and estimate its H–N–H bond angle.
Molecular geometry:
H–N–H bond angle:
Markscheme
partial dissociation «in aqueous solution» [✔]
ethanoic acid/vinegar reacts with NaOH [✔]
moves equilibrium to left/reactant side [✔]
releases Cl2 (g)/chlorine gas
OR
Cl2 (g)/chlorine gas is toxic [✔]
Note: Accept “ethanoic acid produces H+ ions”.
Accept “ethanoic acid/vinegar reacts with NaOCl”.
Do not accept “2CH3COOH + NaOCl + NaCl → 2CH3COONa + Cl2 + H2O” as it does not refer to equilibrium.
Accept suitable molecular or ionic equations for M1 and M3.
[✔]
Note: Accept any combination of dots/crosses or lines to represent electron pairs.
Molecular geometry:
«trigonal» pyramidal [✔]
H–N–H bond angle:
107° [✔]
Note: Accept angles in the range of 100–109.
Examiners report
The definition of a weak acid was generally correct.
Explaining why it was dangerous to mix chlorine with vinegar was not well answered but most students gained at least one mark for stating that “chlorine gas will be produced”, but couldn’t link it to equilibrium ideas.
The Lewis structure of chloramine was correct for strong candidates, but many made the mistake of omitting electron pairs on N and Cl.
The molecular geometry and bond angles often did not correspond to each other with quite a few candidates stating trigonal planar and then 107 for the angle.