| Date | November 2021 | Marks available | 3 | Reference code | 21N.2.hl.TZ0.3 |
| Level | HL | Paper | 2 | Time zone | TZ0 |
| Command term | Deduce | Question number | 3 | Adapted from | N/A |
Question
White phosphorus is an allotrope of phosphorus and exists as P4.
An equilibrium exists between PCl3 and PCl5.
PCl3 (g) + Cl2 (g) PCl5 (g)
Sketch the Lewis (electron dot) structure of the P4 molecule, containing only single bonds.
Write an equation for the reaction of white phosphorus (P4) with chlorine gas to form phosphorus trichloride (PCl3).
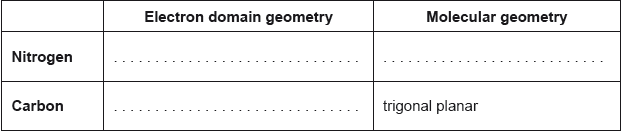
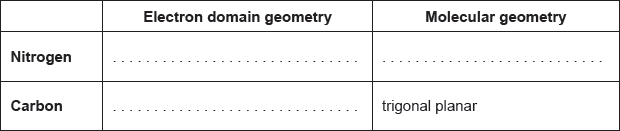
Deduce the electron domain and molecular geometry using VSEPR theory, and estimate the Cl–P–Cl bond angle in PCl3.
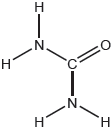
Outline the reason why PCl5 is a non-polar molecule, while PCl4F is polar.
Calculate the standard enthalpy change (ΔH⦵) for the forward reaction in kJ mol−1.
ΔH⦵f PCl3 (g) = −306.4 kJ mol−1
ΔH⦵f PCl5 (g) = −398.9 kJ mol−1
Calculate the entropy change, ΔS, in J K−1 mol−1, for this reaction.
Chemistry 2e, Chpt. 21 Nuclear Chemistry, Appendix G: Standard Thermodynamic Properties for Selected Substances https://openstax.org/books/chemistry-2e/pages/g-standard-thermodynamic-properties-for- selectedsubstances# page_667adccf-f900-4d86-a13d-409c014086ea © 1999-2021, Rice University. Except where otherwise noted, textbooks on this site are licensed under a Creative Commons Attribution 4.0 International License. (CC BY 4.0) https://creativecommons.org/licenses/by/4.0/.
Calculate the Gibbs free energy change (ΔG), in kJ mol−1, for this reaction at 25 °C. Use section 1 of the data booklet.
If you did not obtain an answer in c(i) or c(ii) use −87.6 kJ mol−1 and −150.5 J mol−1 K−1 respectively, but these are not the correct answers.
Determine the equilibrium constant, K, for this reaction at 25 °C, referring to section 1 of the data booklet.
If you did not obtain an answer in (c)(iii), use ΔG = –43.5 kJ mol−1, but this is not the correct answer.
State the equilibrium constant expression, Kc, for this reaction.
State, with a reason, the effect of an increase in temperature on the position of this equilibrium.
Markscheme
Accept any diagram with each P joined to the other three.
Accept any combination of dots, crosses and lines.
P4 (s) + 6Cl2 (g) → 4PCl3 (l) ✔
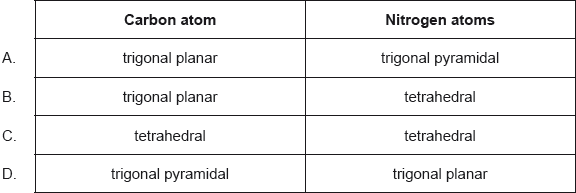
Electron domain geometry: tetrahedral ✔
Molecular geometry: trigonal pyramidal ✔
Bond angle: 100«°» ✔
Accept any value or range within the range 91−108«°» for M3.
PCl5 is non-polar:
symmetrical
OR
dipoles cancel ✔
PCl4F is polar:
P–Cl has a different bond polarity than P–F ✔
non-symmetrical «dipoles»
OR
dipoles do not cancel ✔
Accept F more electronegative than/different electronegativity to Cl for M2.
«−398.9 kJ mol−1 − (−306.4 kJ mol−1) =» −92.5 «kJ mol−1» ✔
«ΔS = 364.5 J K–1 mol–1 – (311.7 J K–1 mol–1 + 223.0 J K–1 mol–1)=» –170.2 «J K–1 mol–1» ✔
«ΔS =» –0.1702 «kJ mol–1 K–1»
OR
298 «K» ✔
«ΔG = –92.5 kJ mol–1 – (298 K × –0.1702 kJ mol–1 K–1) =» –41.8 «kJ mol–1» ✔
Award [2] for correct final answer.
If –87.6 and -150.5 are used then –42.8.
«ΔG = –41.8 kJ mol–1 = × 298 K × lnK»
OR
«ΔG = –41800 J mol–1 = –8.31 J mol–1 K–1 × 298 K × lnK»
«lnK = =» 16.9 ✔
«K = e16.9 =» 2.19 × 107 ✔
Award [2] for correct final answer.
Accept range of 1.80 × 106–2.60 × 107.
If –43.5 is used then 4.25 × 107.
«Kc =» ✔
«shifts» left/towards reactants AND «forward reaction is» exothermic/ΔH is negative ✔