| Date | May 2018 | Marks available | 1 | Reference code | 18M.2.hl.TZ2.7 |
| Level | HL | Paper | 2 | Time zone | TZ2 |
| Command term | Justify and Deduce | Question number | 7 | Adapted from | N/A |
Question
Some physical properties of molecular substances result from the different types of forces between their molecules.
Resonance structures exist when a molecule can be represented by more than one Lewis structure.
Carbon dioxide can be represented by at least two resonance structures, I and II.

Calculate the formal charge on each oxygen atom in the two structures.
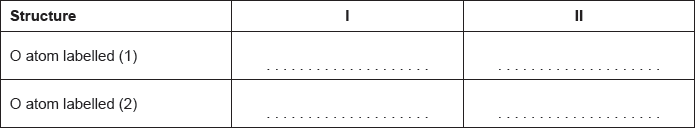
Deduce, giving a reason, the more likely structure.
Absorption of UV light in the ozone layer causes the dissociation of oxygen and ozone.
Identify, in terms of bonding, the molecule that requires a longer wavelength to dissociate.
Carbon and silicon are elements in group 14.
Explain why CO2 is a gas but SiO2 is a solid at room temperature.
Markscheme
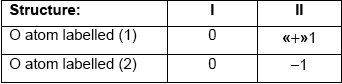
Award [1] for any two correctly filled cells.
[2 marks]
structure I AND no formal charges
OR
structure I AND no charge transfer «between atoms»
[1 mark]
O3 has bond between single and double bond AND O2 has double bond
OR
O3 has bond order of 1.5 AND O2 has bond order of 2
OR
bond in O3 is weaker/longer than in O2
O3 requires longer wavelength
M1: Do not accept “ozone has one single and one double bond”.
[2 marks]
CO2 «non-polar» «weak» London/dispersion forces/instantaneous induced dipole-induced dipole forces between molecules
SiO2 network/lattice/3D/giant «covalent» structure
M1: The concept of “between” is essential.
[2 marks]

