| Date | May 2015 | Marks available | 3 | Reference code | 15M.2.hl.TZ1.1 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | Predict | Question number | 1 | Adapted from | N/A |
Question
Ethanedioic acid is a diprotic acid. A student determined the value of x in the formula of hydrated ethanedioic acid, \({\text{HOOC–COOH}} \bullet {\text{x}}{{\text{H}}_{\text{2}}}{\text{O}}\), by titrating a known mass of the acid with a 0.100 \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of NaOH(aq).
0.795 g of ethanedioic acid was dissolved in distilled water and made up to a total volume of 250 cm3 in a volumetric flask.
\({\text{25 c}}{{\text{m}}^{\text{3}}}\) of this ethanedioic acid solution was pipetted into a flask and titrated against aqueous sodium hydroxide using phenolphthalein as an indicator.
The titration was then repeated twice to obtain the results below.
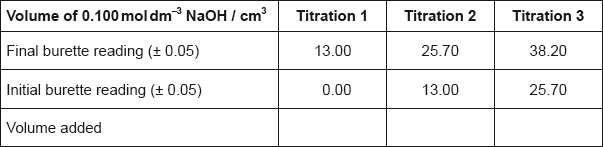
Calculate the average volume of NaOH added, in \({\text{c}}{{\text{m}}^{\text{3}}}\), in titrations 2 and 3, and then calculate the amount, in mol, of NaOH added.
The equation for the reaction taking place in the titration is:
\({\text{HOOC–COOH(aq)}} + {\text{2NaOH(aq)}} \to {\text{NaOOC–COONa(aq)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}}\)
Determine the amount, in mol, of ethanedioic acid that reacts with the average
volume of NaOH(aq).
Determine the amount, in mol, of ethanedioic acid present in \({\text{250 c}}{{\text{m}}^{\text{3}}}\) of the original solution.
Determine the molar mass of hydrated ethanedioic acid.
Determine the value of x in the formula \({\text{HOOC–COOH}} \bullet {\text{x}}{{\text{H}}_{\text{2}}}{\text{O}}\).
Identify the strongest intermolecular force in solid ethanedioic acid.
Deduce the Lewis (electron dot) structure of ethanedioic acid, \({\text{HOOC–COOH}}\).
Predict and explain the difference in carbon-oxygen bond lengths in ethanedioic acid and its conjugate base, \(^ - {\text{OOC–CO}}{{\text{O}}^ - }\).
Markscheme
\(\left( {\frac{{(12.70 + 12.50}}{2} = } \right)12.60{\text{ (c}}{{\text{m}}^3}{\text{);}}\)
\((0.01260 \times 0.100 = )1.26 \times {10^{ - 3}}{\text{ (mol);}}\)
Award [2] for correct final answer.
\(\left( {\frac{{1.26 \times {{10}^{ - 3}}}}{2} = } \right)6.30 \times {10^{ - 4}}{\text{ (mol);}}\)
\((6.30 \times {10^{ - 4}} \times 10 = )6.30 \times {10^{ - 3}}{\text{ (mol);}}\)
\(\left( {\frac{{0.795}}{{6.30 \times {{10}^{ - 3}}}} = } \right)126{\text{ (gmo}}{{\text{l}}^{ - 1}}{\text{);}}\)
\({M_{\text{r}}}{\text{(}}{{\text{C}}_2}{{\text{H}}_2}{{\text{O}}_4}{\text{)}} = 90.04\) and \({M_{\text{r}}}{\text{(}}{{\text{H}}_2}{\text{O)}} = 18.02\);
Accept integer values for \({M_r}\)’s of 90 and 18 and any reasonable calculation.
Award [1 max] if no working shown.
hydrogen bonding;
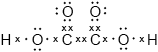 1;
1;
Mark cannot be scored if lone pairs are missing on oxygens.
Accept any combination of lines, dots or crosses to represent electron pairs.
Acid:
one double and one single bond / one shorter and one longer bond;
Accept “two double and two single”.
Conjugate base:
two 1.5 bonds / both bonds same length;
Accept “four / all”.
electrons delocalized / resonance forms;
Award marks for suitable diagrams.
Examiners report
It was suggested that, in the second paragraph, we should have explicitly stated that “0.795 g of hydrated ethanedioic acid was dissolved…” We agree that this would have clarified even more the question but we believe the sense is clear from the actual question for any student with practical experience. Another teacher suggested that the question was too easy. This was not apparent in the answers seen with very few candidates getting all the way to the end without mishap.
Most had little problem with (a) but some averaged all three readings. In (b) candidates found the calculation at the beginning of the paper difficult and many gave up too early in the sequence. “Error carried forward” marks were available even if an error was made early on. In (c), most were able to identify hydrogen bonding successfully. The diagrams of the Lewis structure of ethanedioic in (d) acid were, in general, poor; the most common error was to omit the lone pairs on the O of \({\text{–O–H}}\). Very few candidates were able to give a good explanation of electron delocalization and the differences in bond lengths in ethanedioic acid and the ethanedioate ion. As one respondent suggested, candidates would have benefitted from drawing out the Lewis structure of \({\text{–OOC–CO}}{{\text{O}}^ - }\). We did not ask for this but there was nothing preventing them from doing so.
It was suggested that, in the second paragraph, we should have explicitly stated that “0.795 g of hydrated ethanedioic acid was dissolved…” We agree that this would have clarified even more the question but we believe the sense is clear from the actual question for any student with practical experience. Another teacher suggested that the question was too easy. This was not apparent in the answers seen with very few candidates getting all the way to the end without mishap.
Most had little problem with (a) but some averaged all three readings. In (b) candidates found the calculation at the beginning of the paper difficult and many gave up too early in the sequence. “Error carried forward” marks were available even if an error was made early on. In (c), most were able to identify hydrogen bonding successfully. The diagrams of the Lewis structure of ethanedioic in (d) acid were, in general, poor; the most common error was to omit the lone pairs on the O of \({\text{–O–H}}\). Very few candidates were able to give a good explanation of electron delocalization and the differences in bond lengths in ethanedioic acid and the ethanedioate ion. As one respondent suggested, candidates would have benefitted from drawing out the Lewis structure of \({\text{–OOC–CO}}{{\text{O}}^ - }\). We did not ask for this but there was nothing preventing them from doing so.
It was suggested that, in the second paragraph, we should have explicitly stated that “0.795 g of hydrated ethanedioic acid was dissolved…” We agree that this would have clarified even more the question but we believe the sense is clear from the actual question for any student with practical experience. Another teacher suggested that the question was too easy. This was not apparent in the answers seen with very few candidates getting all the way to the end without mishap.
Most had little problem with (a) but some averaged all three readings. In (b) candidates found the calculation at the beginning of the paper difficult and many gave up too early in the sequence. “Error carried forward” marks were available even if an error was made early on. In (c), most were able to identify hydrogen bonding successfully. The diagrams of the Lewis structure of ethanedioic in (d) acid were, in general, poor; the most common error was to omit the lone pairs on the O of \({\text{–O–H}}\). Very few candidates were able to give a good explanation of electron delocalization and the differences in bond lengths in ethanedioic acid and the ethanedioate ion. As one respondent suggested, candidates would have benefitted from drawing out the Lewis structure of \({\text{–OOC–CO}}{{\text{O}}^ - }\). We did not ask for this but there was nothing preventing them from doing so.
It was suggested that, in the second paragraph, we should have explicitly stated that “0.795 g of hydrated ethanedioic acid was dissolved…” We agree that this would have clarified even more the question but we believe the sense is clear from the actual question for any student with practical experience. Another teacher suggested that the question was too easy. This was not apparent in the answers seen with very few candidates getting all the way to the end without mishap.
Most had little problem with (a) but some averaged all three readings. In (b) candidates found the calculation at the beginning of the paper difficult and many gave up too early in the sequence. “Error carried forward” marks were available even if an error was made early on. In (c), most were able to identify hydrogen bonding successfully. The diagrams of the Lewis structure of ethanedioic in (d) acid were, in general, poor; the most common error was to omit the lone pairs on the O of \({\text{–O–H}}\). Very few candidates were able to give a good explanation of electron delocalization and the differences in bond lengths in ethanedioic acid and the ethanedioate ion. As one respondent suggested, candidates would have benefitted from drawing out the Lewis structure of \({\text{–OOC–CO}}{{\text{O}}^ - }\). We did not ask for this but there was nothing preventing them from doing so.
It was suggested that, in the second paragraph, we should have explicitly stated that “0.795 g of hydrated ethanedioic acid was dissolved…” We agree that this would have clarified even more the question but we believe the sense is clear from the actual question for any student with practical experience. Another teacher suggested that the question was too easy. This was not apparent in the answers seen with very few candidates getting all the way to the end without mishap.
Most had little problem with (a) but some averaged all three readings. In (b) candidates found the calculation at the beginning of the paper difficult and many gave up too early in the sequence. “Error carried forward” marks were available even if an error was made early on. In (c), most were able to identify hydrogen bonding successfully. The diagrams of the Lewis structure of ethanedioic in (d) acid were, in general, poor; the most common error was to omit the lone pairs on the O of \({\text{–O–H}}\). Very few candidates were able to give a good explanation of electron delocalization and the differences in bond lengths in ethanedioic acid and the ethanedioate ion. As one respondent suggested, candidates would have benefitted from drawing out the Lewis structure of \({\text{–OOC–CO}}{{\text{O}}^ - }\). We did not ask for this but there was nothing preventing them from doing so.
It was suggested that, in the second paragraph, we should have explicitly stated that “0.795 g of hydrated ethanedioic acid was dissolved…” We agree that this would have clarified even more the question but we believe the sense is clear from the actual question for any student with practical experience. Another teacher suggested that the question was too easy. This was not apparent in the answers seen with very few candidates getting all the way to the end without mishap.
Most had little problem with (a) but some averaged all three readings. In (b) candidates found the calculation at the beginning of the paper difficult and many gave up too early in the sequence. “Error carried forward” marks were available even if an error was made early on. In (c), most were able to identify hydrogen bonding successfully. The diagrams of the Lewis structure of ethanedioic in (d) acid were, in general, poor; the most common error was to omit the lone pairs on the O of \({\text{–O–H}}\). Very few candidates were able to give a good explanation of electron delocalization and the differences in bond lengths in ethanedioic acid and the ethanedioate ion. As one respondent suggested, candidates would have benefitted from drawing out the Lewis structure of \({\text{–OOC–CO}}{{\text{O}}^ - }\). We did not ask for this but there was nothing preventing them from doing so.
It was suggested that, in the second paragraph, we should have explicitly stated that “0.795 g of hydrated ethanedioic acid was dissolved…” We agree that this would have clarified even more the question but we believe the sense is clear from the actual question for any student with practical experience. Another teacher suggested that the question was too easy. This was not apparent in the answers seen with very few candidates getting all the way to the end without mishap.
Most had little problem with (a) but some averaged all three readings. In (b) candidates found the calculation at the beginning of the paper difficult and many gave up too early in the sequence. “Error carried forward” marks were available even if an error was made early on. In (c), most were able to identify hydrogen bonding successfully. The diagrams of the Lewis structure of ethanedioic in (d) acid were, in general, poor; the most common error was to omit the lone pairs on the O of \({\text{–O–H}}\). Very few candidates were able to give a good explanation of electron delocalization and the differences in bond lengths in ethanedioic acid and the ethanedioate ion. As one respondent suggested, candidates would have benefitted from drawing out the Lewis structure of \({\text{–OOC–CO}}{{\text{O}}^ - }\). We did not ask for this but there was nothing preventing them from doing so.
It was suggested that, in the second paragraph, we should have explicitly stated that “0.795 g of hydrated ethanedioic acid was dissolved…” We agree that this would have clarified even more the question but we believe the sense is clear from the actual question for any student with practical experience. Another teacher suggested that the question was too easy. This was not apparent in the answers seen with very few candidates getting all the way to the end without mishap.
Most had little problem with (a) but some averaged all three readings. In (b) candidates found the calculation at the beginning of the paper difficult and many gave up too early in the sequence. “Error carried forward” marks were available even if an error was made early on. In (c), most were able to identify hydrogen bonding successfully. The diagrams of the Lewis structure of ethanedioic in (d) acid were, in general, poor; the most common error was to omit the lone pairs on the O of \({\text{–O–H}}\). Very few candidates were able to give a good explanation of electron delocalization and the differences in bond lengths in ethanedioic acid and the ethanedioate ion. As one respondent suggested, candidates would have benefitted from drawing out the Lewis structure of \({\text{–OOC–CO}}{{\text{O}}^ - }\). We did not ask for this but there was nothing preventing them from doing so.

