| Date | May 2011 | Marks available | 1 | Reference code | 11M.1.hl.TZ1.14 |
| Level | HL | Paper | 1 | Time zone | TZ1 |
| Command term | Deduce | Question number | 14 | Adapted from | N/A |
Question
The Lewis structure of \({\text{S}}{{\text{O}}_{\text{2}}}\) is given below.
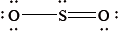
What is the shape of the \({\text{S}}{{\text{O}}_{\text{2}}}\) molecule?
A. Bent (V-shaped)
B. Linear
C. T-shaped
D. Triangular planar
Markscheme
A
Examiners report
There were two G2 comments on this question. One respondent stated that D. should be trigonal planar instead of triangular planar. Both terms are widely used in fact, though of course the correct answer is A. bent or V-shaped. Another respondent stated that it would have been better to represent the Lewis structure of \({\text{S}}{{\text{O}}_{\text{2}}}\) with valence expansion. It is true that \({\text{S}}{{\text{O}}_{\text{2}}}\) could be represented as an alternate Lewis structure. However, the question did not state what the best Lewis structure representation of \({\text{S}}{{\text{O}}_{\text{2}}}\) was and hence was not basing the representation at any distinction centred on formal charge differences versus expanded octets. Candidates simply had to look at the three negative charge centres present which equates to a triangular planar electron-domain geometry and hence a bent molecular geometry as the final shape giving A as the correct answer.

