| Date | May 2013 | Marks available | 1 | Reference code | 13M.2.hl.TZ2.5 |
| Level | HL | Paper | 2 | Time zone | TZ2 |
| Command term | Predict | Question number | 5 | Adapted from | N/A |
Question
Phosphoryl chloride, \({\text{POC}}{{\text{l}}_{\text{3}}}\), is a dehydrating agent.
\({\text{POC}}{{\text{l}}_{\text{3}}}\left( {\text{g}} \right)\) decomposes according to the following equation.
\[{\text{2POC}}{{\text{l}}_3}{\text{(g)}} \to {\text{2PC}}{{\text{l}}_3}{\text{(g)}} + {{\text{O}}_2}{\text{(g)}}\]
POCl3 can be prepared by the reaction of phosphorus pentachloride, PCl5 , with tetraphosphorus decaoxide, P4O10.
PCl3 and Cl– can act as ligands in transition metal complexes such as Ni(PCl3)4 and [Cr(H2O)3Cl3].
Predict and explain the sign of the entropy change, \(\Delta S\), for this reaction.
Calculate the standard entropy change for the reaction, \(\Delta {S^\Theta }\), in \({\text{J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}\), using the data below.
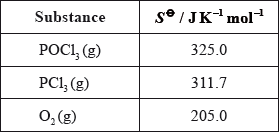
Define the term standard enthalpy change of formation, \(\Delta H_{\text{f}}^\Theta \).
Calculate the standard enthalpy change for the reaction, \(\Delta {H^\Theta }\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), using the data below.
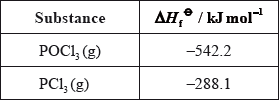
Determine the standard free energy change for the reaction, \(\Delta {G^\Theta }\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), at 298 K.
Deduce the temperature, in K, at which the reaction becomes spontaneous.
Deduce the Lewis (electron dot) structure of POCl3 (with P as the central element) and PCl3 and predict the shape of each molecule, using the valence shell electron pair repulsion theory (VSEPR).
State and explain the Cl–P–Cl bond angle in PCl3.
Deduce the Lewis (electron dot) structure of PCl5.
Predict the shape of this molecule, using the valence shell electron pair repulsion theory (VSEPR).
Identify all the different bond angles in PCl5.
PCl3Br2 has the same molecular shape as PCl5. Draw the three isomers of PCl3Br2 and deduce whether each isomer is polar or non-polar.
Define the term ligand.
Explain why the complex [Cr(H2O)3Cl3] is coloured.
Markscheme
2 mol (g) going to 3 mol (g)/increase in number of particles, therefore entropy increases/\(\Delta S\) positive / OWTTE;
Accept if numbers of moles of gas are given below the equation.
\(\left( {\Delta {S^\Theta } = [(2)(311.7) + (205.0)] - (2)(325.0) = } \right)( + )178.4{\text{ }}({\text{J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}})\);
heat/enthalpy change/required/absorbed when 1 mol of a compound is formed from its elements in their standard states/at 100 kPa/105 Pa/1 bar;
Allow 1.01 \( \times \) 105 Pa/101 kPa/1 atm.
Allow under standard conditions or standard temperature and pressure.
Temperatures not required in definition, allow if quoted (for example, 298 K/ 25 °C – most common) but pressure value must be correct if stated.
\(\left( {\Delta {H^\Theta } = [(2)( - 288.1)] - [(2)( - 542.2)]) = } \right)( + )508.2{\text{ }}({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
\(\left( {\Delta {G^\Theta } = \Delta {H^\Theta } - T\Delta {S^\Theta } = (508.2) - (298)\left( {\frac{{178.4}}{{1000}}} \right) = } \right){\text{ ( + )}}455.0{\text{ }}({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
\(T > \left( {\frac{{\Delta {H^\Theta }}}{{\Delta {S^\Theta }}} = \frac{{508.2}}{{\left( {\frac{{178.4}}{{1000}}} \right)}} = } \right){\text{ }}2849{\text{ (K)}}/2576\) (°C);
Allow temperatures in the range 2848–2855 K.
Accept T = 2849(K) .
No ECF for temperatures T in the range 0–100 K.
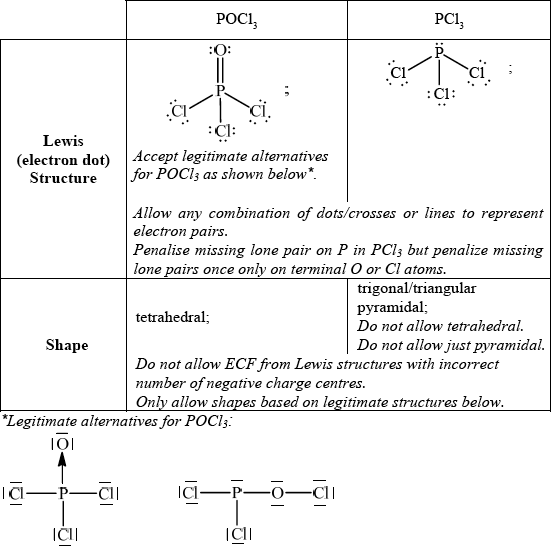
allow any bond angle in the range 100° to less than 109° (experimental value is100°);
due to four negative charge centres/four electron pairs/four electron domains (one of which is a lone pair)/tetrahedral arrangement of electron pairs/domains;
extra repulsion due to lone pair electrons / lone pairs occupy more space (than bonding pairs) so Cl–P–Cl bond angle decreases from 109.5° / OWTTE;
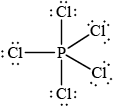 ;
;
Allow any combination of dots/crosses or lines to represent electron pairs.
Do not penalise missing lone pairs on Cl if already penalised in (b)(i).
trigonal/triangular bipyramidal;
Do not allow ECF from Lewis structures with incorrect number of negative charge centres.
120° and 90°/180°;
Ignore other bond angles such as 240° and 360°.
Apply list principle if some correct and incorrect angles given.
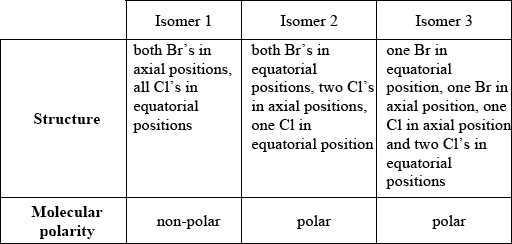
Award [1] for correct structure and molecular polarity.
Award [1 max] for correct representations of all three isomers.
Lone pairs not required.
species with lone/non-bonding pair (of electrons);
which bonds to metal ion (in complex) / which forms dative (covalent)/coordinate bond to metal ion (in complex);
unpaired electrons in d orbitals / d sub-level partially occupied;
d orbitals split (into two sets of different energies);
frequencies of (visible) light absorbed by electrons moving from lower to higher d levels;
colour due to remaining frequencies / complementary colour transmitted;
Allow wavelength as well as frequency.
Do not accept colour emitted.
Examiners report
Most candidates were able to calculate the entropy, enthalpy and free energy changes but made mistakes with the correct definition of enthalpy of formation’. Many referred to the gaseous state which suggests some confusion with bond enthalpies. Many were comfortable with writing Lewis structures and shapes of molecules, or some give incomplete explanations, not referring to the number of electron domains for example. Not many students could write a balanced equation for the reaction between PCl3 and H2O (A.S. 13.1.2 of the guide). In part (d) even though many knew that a ligand has a lone pair of electrons, they missed the second mark for ‘bonding to metal ion’.
Most candidates were able to calculate the entropy, enthalpy and free energy changes but made mistakes with the correct definition of enthalpy of formation’. Many referred to the gaseous state which suggests some confusion with bond enthalpies. Many were comfortable with writing Lewis structures and shapes of molecules, or some give incomplete explanations, not referring to the number of electron domains for example. Not many students could write a balanced equation for the reaction between PCl3 and H2O (A.S. 13.1.2 of the guide). In part (d) even though many knew that a ligand has a lone pair of electrons, they missed the second mark for ‘bonding to metal ion’.
Most candidates were able to calculate the entropy, enthalpy and free energy changes but made mistakes with the correct definition of enthalpy of formation’. Many referred to the gaseous state which suggests some confusion with bond enthalpies. Many were comfortable with writing Lewis structures and shapes of molecules, or some give incomplete explanations, not referring to the number of electron domains for example. Not many students could write a balanced equation for the reaction between PCl3 and H2O (A.S. 13.1.2 of the guide). In part (d) even though many knew that a ligand has a lone pair of electrons, they missed the second mark for ‘bonding to metal ion’.
Most candidates were able to calculate the entropy, enthalpy and free energy changes but made mistakes with the correct definition of enthalpy of formation’. Many referred to the gaseous state which suggests some confusion with bond enthalpies. Many were comfortable with writing Lewis structures and shapes of molecules, or some give incomplete explanations, not referring to the number of electron domains for example. Not many students could write a balanced equation for the reaction between PCl3 and H2O (A.S. 13.1.2 of the guide). In part (d) even though many knew that a ligand has a lone pair of electrons, they missed the second mark for ‘bonding to metal ion’.
Most candidates were able to calculate the entropy, enthalpy and free energy changes but made mistakes with the correct definition of enthalpy of formation’. Many referred to the gaseous state which suggests some confusion with bond enthalpies. Many were comfortable with writing Lewis structures and shapes of molecules, or some give incomplete explanations, not referring to the number of electron domains for example. Not many students could write a balanced equation for the reaction between PCl3 and H2O (A.S. 13.1.2 of the guide). In part (d) even though many knew that a ligand has a lone pair of electrons, they missed the second mark for ‘bonding to metal ion’.
Most candidates were able to calculate the entropy, enthalpy and free energy changes but made mistakes with the correct definition of enthalpy of formation’. Many referred to the gaseous state which suggests some confusion with bond enthalpies. Many were comfortable with writing Lewis structures and shapes of molecules, or some give incomplete explanations, not referring to the number of electron domains for example. Not many students could write a balanced equation for the reaction between PCl3 and H2O (A.S. 13.1.2 of the guide). In part (d) even though many knew that a ligand has a lone pair of electrons, they missed the second mark for ‘bonding to metal ion’.
Most candidates were able to calculate the entropy, enthalpy and free energy changes but made mistakes with the correct definition of enthalpy of formation’. Many referred to the gaseous state which suggests some confusion with bond enthalpies. Many were comfortable with writing Lewis structures and shapes of molecules, or some give incomplete explanations, not referring to the number of electron domains for example. Not many students could write a balanced equation for the reaction between PCl3 and H2O (A.S. 13.1.2 of the guide). In part (d) even though many knew that a ligand has a lone pair of electrons, they missed the second mark for ‘bonding to metal ion’.
Most candidates were able to calculate the entropy, enthalpy and free energy changes but made mistakes with the correct definition of enthalpy of formation’. Many referred to the gaseous state which suggests some confusion with bond enthalpies. Many were comfortable with writing Lewis structures and shapes of molecules, or some give incomplete explanations, not referring to the number of electron domains for example. Not many students could write a balanced equation for the reaction between PCl3 and H2O (A.S. 13.1.2 of the guide). In part (d) even though many knew that a ligand has a lone pair of electrons, they missed the second mark for ‘bonding to metal ion’.
Most candidates were able to calculate the entropy, enthalpy and free energy changes but made mistakes with the correct definition of enthalpy of formation’. Many referred to the gaseous state which suggests some confusion with bond enthalpies. Many were comfortable with writing Lewis structures and shapes of molecules, or some give incomplete explanations, not referring to the number of electron domains for example. Not many students could write a balanced equation for the reaction between PCl3 and H2O (A.S. 13.1.2 of the guide). In part (d) even though many knew that a ligand has a lone pair of electrons, they missed the second mark for ‘bonding to metal ion’.
Most candidates were able to calculate the entropy, enthalpy and free energy changes but made mistakes with the correct definition of enthalpy of formation’. Many referred to the gaseous state which suggests some confusion with bond enthalpies. Many were comfortable with writing Lewis structures and shapes of molecules, or some give incomplete explanations, not referring to the number of electron domains for example. Not many students could write a balanced equation for the reaction between PCl3 and H2O (A.S. 13.1.2 of the guide). In part (d) even though many knew that a ligand has a lone pair of electrons, they missed the second mark for ‘bonding to metal ion’.
Most candidates were able to calculate the entropy, enthalpy and free energy changes but made mistakes with the correct definition of enthalpy of formation’. Many referred to the gaseous state which suggests some confusion with bond enthalpies. Many were comfortable with writing Lewis structures and shapes of molecules, or some give incomplete explanations, not referring to the number of electron domains for example. Not many students could write a balanced equation for the reaction between PCl3 and H2O (A.S. 13.1.2 of the guide). In part (d) even though many knew that a ligand has a lone pair of electrons, they missed the second mark for ‘bonding to metal ion’.
Most candidates were able to calculate the entropy, enthalpy and free energy changes but made mistakes with the correct definition of enthalpy of formation’. Many referred to the gaseous state which suggests some confusion with bond enthalpies. Many were comfortable with writing Lewis structures and shapes of molecules, or some give incomplete explanations, not referring to the number of electron domains for example. Not many students could write a balanced equation for the reaction between PCl3 and H2O (A.S. 13.1.2 of the guide). In part (d) even though many knew that a ligand has a lone pair of electrons, they missed the second mark for ‘bonding to metal ion’.
Most candidates were able to calculate the entropy, enthalpy and free energy changes but made mistakes with the correct definition of enthalpy of formation’. Many referred to the gaseous state which suggests some confusion with bond enthalpies. Many were comfortable with writing Lewis structures and shapes of molecules, or some give incomplete explanations, not referring to the number of electron domains for example. Not many students could write a balanced equation for the reaction between PCl3 and H2O (A.S. 13.1.2 of the guide). In part (d) even though many knew that a ligand has a lone pair of electrons, they missed the second mark for ‘bonding to metal ion’.
Most candidates were able to calculate the entropy, enthalpy and free energy changes but made mistakes with the correct definition of enthalpy of formation’. Many referred to the gaseous state which suggests some confusion with bond enthalpies. Many were comfortable with writing Lewis structures and shapes of molecules, or some give incomplete explanations, not referring to the number of electron domains for example. Not many students could write a balanced equation for the reaction between PCl3 and H2O (A.S. 13.1.2 of the guide). In part (d) even though many knew that a ligand has a lone pair of electrons, they missed the second mark for ‘bonding to metal ion’.

