| Date | May 2013 | Marks available | 2 | Reference code | 13M.2.hl.TZ1.6 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | Deduce | Question number | 6 | Adapted from | N/A |
Question
The element boron has two naturally occurring isotopes, \(^{{\text{10}}}{\text{B}}\) and \(^{{\text{11}}}{\text{B}}\).
Phosphorus forms two chlorides, \({\text{PC}}{{\text{l}}_{\text{3}}}\) and \({\text{PC}}{{\text{l}}_{\text{5}}}\).
Apply the Aufbau principle to state the full electron configuration for an atom of phosphorus.
Deduce the Lewis structures for \({\text{PC}}{{\text{l}}_{\text{3}}}\) and \({\text{PC}}{{\text{l}}_{\text{5}}}\).
\({\text{PC}}{{\text{l}}_{\text{3}}}\)\(\quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \)\({\text{PC}}{{\text{l}}_{\text{5}}}\)
Predict the shapes and the bond angles in the two molecules.
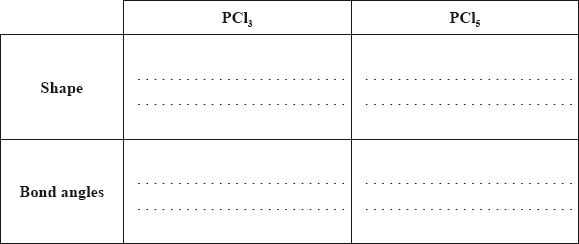
Identify the type of hybridization present in \({\text{PC}}{{\text{l}}_{\text{3}}}\).
Compare the melting points of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and \({\text{PC}}{{\text{l}}_{\text{5}}}\) and explain the difference.
Define an acid according to the Lewis theory.
State and explain the acid–base character of \({\text{PC}}{{\text{l}}_{\text{3}}}\) according to the Lewis theory.
Explain the delocalization of \(\pi \) electrons using the \({{\text{O}}_{\text{3}}}\) molecule as an example, including two facts that support the delocalization.
Markscheme
\({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{3}}}\);
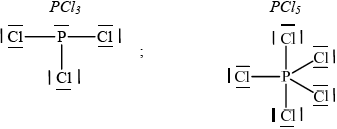 ;
;
Penalize missing lone pairs on chlorine only once.
Accept any combination of lines, dots or crosses to represent electron pairs.

Shape and bond angle must be consistent with the number of electron domains given in the diagram in (ii).
\({\text{s}}{{\text{p}}^{\text{3}}}\) (hybridization);
\({\text{PC}}{{\text{l}}_{\text{5}}}\) has higher melting point than \({\text{PC}}{{\text{l}}_{\text{3}}}\);
\({\text{PC}}{{\text{l}}_{\text{5}}}\) has stronger intermolecular/London/dispersion/van der Waals’ forces;
(because of) more electrons/greater mass;
Accept the opposite argument for PCl3.
Award [1 max] for answers suggesting PCl3 has higher melting point because it is polar and PCl5 is not.
electron pair acceptor;
Lewis base;
has non-bonding/lone pair of electrons;
No ECF from (i).
overlap of \(p\) orbitals / \(p\) electrons of double/\(\pi \) bond and non-bonding/lone pair on oxygen interact / OWTTE;
\(\pi \) electrons not localized / different resonance structures possible /
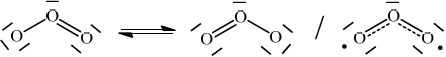 ;
;
both bonds/O–O and O=O have equal length / OWTTE;
both bonds/O–O and O=O have equal bond energy / OWTTE;
Examiners report
The electron configuration of phosphorus was successfully answered (even by apparently weaker candidates) and there were many good answers for the Lewis structures. Candidates would do well to draw the “dots” clearly remembering that their answer will be scanned. They should group the electron dots neatly in pairs (much easier for the examiner to count, for one thing) or use a line to represent an electron pair. The usual errors occurred namely missing lone pairs on P and/or Cl atoms.
The electron configuration of phosphorus was successfully answered (even by apparently weaker candidates) and there were many good answers for the Lewis structures. Candidates would do well to draw the “dots” clearly remembering that their answer will be scanned. They should group the electron dots neatly in pairs (much easier for the examiner to count, for one thing) or use a line to represent an electron pair. The usual errors occurred namely missing lone pairs on P and/or Cl atoms.
The shapes and angles in (iii) were patchy but there were also some impressive answers.
About half knew that \({\text{s}}{{\text{p}}^{\text{3}}}\) was the answer to (iv) and in (v) about half based their explanation on the dipole moment in \({\text{PC}}{{\text{l}}_{\text{3}}}\). (One mark was allowed for those who recognized that \({\text{PC}}{{\text{l}}_{\text{3}}}\) would be polar whilst \({\text{PC}}{{\text{l}}_{\text{5}}}\) would not – thus suggesting that \({\text{PC}}{{\text{l}}_{\text{3}}}\) had the higher melting point.) Candidates were expected to know the order of melting points as this had been studied in 13.1.1. Very few were able to write a balanced equation for the reaction of \({\text{PC}}{{\text{l}}_{\text{5}}}\) with water.
About half knew that \({\text{s}}{{\text{p}}^{\text{3}}}\) was the answer to (iv) and in (v) about half based their explanation on the dipole moment in \({\text{PC}}{{\text{l}}_{\text{3}}}\). (One mark was allowed for those who recognized that \({\text{PC}}{{\text{l}}_{\text{3}}}\) would be polar whilst \({\text{PC}}{{\text{l}}_{\text{5}}}\) would not – thus suggesting that \({\text{PC}}{{\text{l}}_{\text{3}}}\) had the higher melting point.) Candidates were expected to know the order of melting points as this had been studied in 13.1.1. Very few were able to write a balanced equation for the reaction of \({\text{PC}}{{\text{l}}_{\text{5}}}\) with water.
Many failed to note that a Lewis acid is an electron pair acceptor and the definition was often muddled with that of Brønsted-Lowry.
Some, in (d) (ii), treated the P and Cl atoms separately.
In (e) there was little discussion of overlap of \(p\) orbitals, some of resonance but hardly any evidence in terms of equal bond length and equal bond strength. The bonding in an ozone molecule was not well-understood.

