| Date | May 2018 | Marks available | 1 | Reference code | 18M.2.hl.TZ1.1 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | Suggest | Question number | 1 | Adapted from | N/A |
Question
Urea, (H2N)2CO, is excreted by mammals and can be used as a fertilizer.
Urea can also be made by the direct combination of ammonia and carbon dioxide gases.
2NH3(g) + CO2(g) \( \rightleftharpoons \) (H2N)2CO(g) + H2O(g) ΔH < 0
Calculate the percentage by mass of nitrogen in urea to two decimal places using section 6 of the data booklet.
Suggest how the percentage of nitrogen affects the cost of transport of fertilizers giving a reason.
The structural formula of urea is shown.
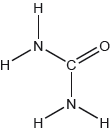
Predict the electron domain and molecular geometries at the nitrogen and carbon atoms, applying the VSEPR theory.
Urea can be made by reacting potassium cyanate, KNCO, with ammonium chloride, NH4Cl.
KNCO(aq) + NH4Cl(aq) → (H2N)2CO(aq) + KCl(aq)
Determine the maximum mass of urea that could be formed from 50.0 cm3 of 0.100 mol dm−3 potassium cyanate solution.
State the equilibrium constant expression, Kc.
Predict, with a reason, the effect on the equilibrium constant, Kc, when the temperature is increased.
Determine an approximate order of magnitude for Kc, using sections 1 and 2 of the data booklet. Assume ΔGΘ for the forward reaction is approximately +50 kJ at 298 K.
Suggest one reason why urea is a solid and ammonia a gas at room temperature.
Sketch two different hydrogen bonding interactions between ammonia and water.
The combustion of urea produces water, carbon dioxide and nitrogen.
Formulate a balanced equation for the reaction.
Calculate the maximum volume of CO2, in cm3, produced at STP by the combustion of 0.600 g of urea, using sections 2 and 6 of the data booklet.
Describe the bond formation when urea acts as a ligand in a transition metal complex ion.
The C–N bonds in urea are shorter than might be expected for a single C–N bond. Suggest, in terms of electrons, how this could occur.
The mass spectrum of urea is shown below.
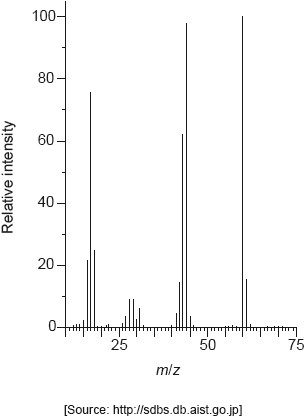
Identify the species responsible for the peaks at m/z = 60 and 44.
The IR spectrum of urea is shown below.
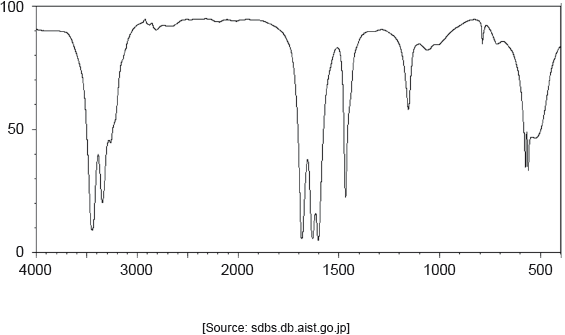
Identify the bonds causing the absorptions at 3450 cm−1 and 1700 cm−1 using section 26 of the data booklet.
Predict the number of signals in the 1H NMR spectrum of urea.
Predict the splitting pattern of the 1H NMR spectrum of urea.
Outline why TMS (tetramethylsilane) may be added to the sample to carry out 1H NMR spectroscopy and why it is particularly suited to this role.
Markscheme
molar mass of urea «4 \( \times \) 1.01 + 2 \( \times \) 14.01 + 12.01 + 16.00» = 60.07 «g mol-1»
«% nitrogen = \(\frac{{{\text{2}} \times {\text{14.01}}}}{{{\text{60.07}}}}\) \( \times \) 100 =» 46.65 «%»
Award [2] for correct final answer.
Award [1 max] for final answer not to two decimal places.
[2 marks]
«cost» increases AND lower N% «means higher cost of transportation per unit of nitrogen»
OR
«cost» increases AND inefficient/too much/about half mass not nitrogen
Accept other reasonable explanations.
Do not accept answers referring to safety/explosions.
[1 mark]

Note: Urea’s structure is more complex than that predicted from VSEPR theory.
[3 marks]
n(KNCO) «= 0.0500 dm3 \( \times \) 0.100 mol dm–3» = 5.00 \( \times \) 10–3 «mol»
«mass of urea = 5.00 \( \times \) 10–3 mol \( \times \) 60.07 g mol–1» = 0.300 «g»
Award [2] for correct final answer.
[2 marks]
\({K_{\text{c}}} = \frac{{[{{({{\text{H}}_2}{\text{N}})}_2}{\text{CO}}] \times [{{\text{H}}_2}{\text{O}}]}}{{{{[{\text{N}}{{\text{H}}_3}]}^2} \times [{\text{C}}{{\text{O}}_2}]}}\)
[1 mark]
«Kc» decreases AND reaction is exothermic
OR
«Kc» decreases AND ΔH is negative
OR
«Kc» decreases AND reverse/endothermic reaction is favoured
[1 mark]
ln K « = \(\frac{{ - \Delta {G^\Theta }}}{{RT}} = \frac{{ - 50 \times {{10}^3}{\text{ J}}}}{{8.31{\text{ J }}{{\text{K}}^{ - 1}}{\text{ mo}}{{\text{l}}^{ - 1}} \times 298{\text{ K}}}}\) » = –20
«Kc =» 2 \( \times \) 10–9
OR
1.69 \( \times \) 10–9
OR
10–9
Accept range of 20-20.2 for M1.
Award [2] for correct final answer.
[2 marks]
Any one of:
urea has greater molar mass
urea has greater electron density/greater London/dispersion
urea has more hydrogen bonding
urea is more polar/has greater dipole moment
Accept “urea has larger size/greater van der Waals forces”.
Do not accept “urea has greater intermolecular forces/IMF”.
[1 mark]
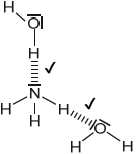
Award [1] for each correct interaction.
If lone pairs are shown on N or O, then the lone pair on N or one of the lone pairs on O MUST be involved in the H-bond.
Penalize solid line to represent H-bonding only once.
[2 marks]
2(H2N)2CO(s) + 3O2(g) → 4H2O(l) + 2CO2(g) + 2N2(g)
correct coefficients on LHS
correct coefficients on RHS
Accept (H2N)2CO(s) + \(\frac{3}{2}\)O2(g) → 2H2O(l) + CO2(g) + N2(g).
Accept any correct ratio.
[2 marks]
«V = \(\frac{{{\text{0.600 g}}}}{{{\text{60.07 g mo}}{{\text{l}}^{ - 1}}}}\) \( \times \) 22700 cm3 mol–1 =» 227 «cm3»
[1 mark]
lone/non-bonding electron pairs «on nitrogen/oxygen/ligand» given to/shared with metal ion
co-ordinate/dative/covalent bonds
[2 marks]
lone pairs on nitrogen atoms can be donated to/shared with C–N bond
OR
C–N bond partial double bond character
OR
delocalization «of electrons occurs across molecule»
OR
slight positive charge on C due to C=O polarity reduces C–N bond length
[1 mark]
60: CON2H4+
44: CONH2+
Accept “molecular ion”.
[2 marks]
3450 cm–1: N–H
1700 cm–1: C=O
Do not accept “O–H” for 3450 cm–1.
[2 marks]
1
[2 marks]
singlet
Accept “no splitting”.
[1 mark]
acts as internal standard
OR
acts as reference point
one strong signal
OR
12 H atoms in same environment
OR
signal is well away from other absorptions
Accept “inert” or “readily removed” or “non-toxic” for M1.
[2 marks]

