| Date | May 2012 | Marks available | 6 | Reference code | 12M.2.hl.TZ2.4 |
| Level | HL | Paper | 2 | Time zone | TZ2 |
| Command term | Draw | Question number | 4 | Adapted from | N/A |
Question
Draw the Lewis structures, predict the shape and deduce the bond angles for xenon tetrafluoride and the nitrate ion.
Markscheme
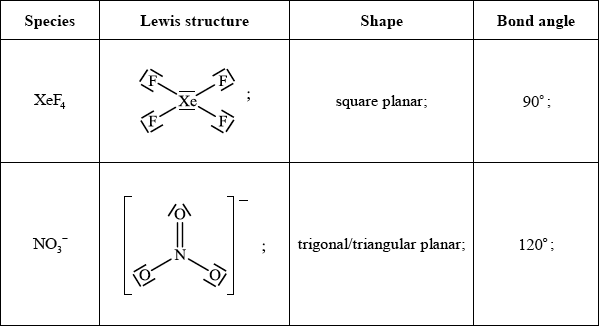
Accept any combination of lines, dots or crosses to represent electron pairs.
Examiners report
Many candidates had difficulties with the Lewis structure of the nitrate ion, putting too many electrons around the nitrogen atom or omitting the negative charge. They did better with XeF4, giving correct shape and bond angles. Some candidates lost marks for omitting lone pairs from the F atoms, and some had only 4 negative charge centres around Xe. Some teachers expressed concern that only exceptions to rules were being examined, but both \({\text{NO}}_3^ - \) and \({\text{Xe}}{{\text{F}}_{\text{4}}}\) are listed as examples to use in the teacher‘s notes.
Syllabus sections
- 17N.2.hl.TZ0.4a: Draw the Lewis (electron dot) structures of PF3 and PF5 and use the VSEPR theory to deduce...
- 17N.1.hl.TZ0.14: How many sigma (σ) and pi (π) bonds are present in this molecule?
- 17M.2.hl.TZ2.7c.ii: Outline, in terms of the bonding present, why the reaction conditions of halogenation are...
- 17M.2.hl.TZ2.4b.ii: Deduce one resonance structure of ozone and the corresponding formal charges on each oxygen...
- 17M.2.hl.TZ2.4b.i: Discuss the bonding in the resonance structures of ozone.
- 17M.2.hl.TZ2.3b: Deduce the Lewis (electron dot) structure and molecular geometry and the bond angles of PCl3.
- 17M.1.hl.TZ2.13: Which statement is correct? A. Sigma bonds are formed only by the combination of s...
- 17M.1.hl.TZ2.10: Which does not show resonance? A. PO43– B. C6H6 C. C6H12 D. O3
- 17M.2.hl.TZ1.8b: Suggest why the loss of ozone is an international environmental concern.
- 17M.2.hl.TZ1.8a: Formulate two equations to show how nitrogen(II) oxide, NO, catalyses the destruction of ozone.
- 17M.1.hl.TZ1.13: Which species have resonance structures? I. Ozone, O3II. Carbon dioxide, CO2III. ...
- 17M.1.hl.TZ1.12: Which combination describes the bonding and structure in benzoic acid, C6H5COOH?
- 17M.1.hl.TZ1.11: Which combination describes the PH4+ ion?
- 16N.3.hl.TZ0.22c: (i) Uranium hexafluoride, UF6, is used in the uranium enrichment process that produces fuel...
- 16N.2.hl.TZ0.5b: (i) Draw diagrams to show how sigma (σ) and pi (π) bonds are formed between atoms. (ii)...
- 16N.1.hl.TZ0.15: What is the hybridization of the numbered atoms in ethanoic acid?
- 16N.1.hl.TZ0.14: Which species has bond angles of 90°? A. AlCl4- B. \({\text{I}}\)Cl4- C. NH4+ D. SiCl4
- 16M.2.hl.TZ0.3c: One of the intermediates in the reaction between nitrogen monoxide and hydrogen is dinitrogen...
- 16M.1.hl.TZ0.14: In which group do both compounds contain delocalized...
- 16M.1.hl.TZ0.11: Which species breaks the octet rule? A. PCl3B. BF4−C. SCl4D....
- 15M.1.hl.TZ1.12: What is correct for \({\text{PC}}{{\text{l}}_{\text{5}}}\)?
- 15M.1.hl.TZ2.12: Which combination of shape and bond angle is correct for a molecule of xenon tetrafluoride,...
- 15M.2.hl.TZ1.1e: Predict and explain the difference in carbon-oxygen bond lengths in ethanedioic acid and its...
- 15M.2.hl.TZ1.7f: Describe the formation of \(\sigma \) and \(\pi \) bonds in an alkene.
- 15M.2.hl.TZ2.9c.i: Deduce the Lewis (electron dot) structure of both molecules.
- 15M.2.hl.TZ2.9c.ii: Predict the shapes of the two molecules, giving the Br–P–Br bond angle in...
- 15M.2.hl.TZ2.9c.iii: Explain why both \({\text{PB}}{{\text{r}}_{\text{3}}}\) and...
- 15M.2.hl.TZ2.9d.iii: Describe sigma \((\sigma )\) and pi \((\pi )\) bonds between atoms. \(\sigma \)...
- 15M.2.hl.TZ2.9d.iv: Identify the number of sigma \((\sigma )\) and pi \((\pi )\) bonds present in a molecule of...
- 15M.3.hl.TZ1.20a: Explain, on a molecular level, why ozone dissociates with radiation of a longer wavelength...
- 15M.3.hl.TZ1.20b: Nitrogen(II) oxide, NO, is a primary pollutant that depletes the ozone layer. State...
- 15M.3.hl.TZ2.23b.ii: Identify and state the source of one other ozone-depleting pollutant.
- 15M.3.hl.TZ2.23b.i: Describe, by means of equations, how nitrogen(II) oxide, NO, catalyses the depletion of ozone.
- 14M.1.hl.TZ1.13: Which species contain delocalized electrons? A. I and II only B. I and III...
- 14M.1.hl.TZ1.12: The diagrams below show \(s\) and \(p\) orbitals in different positions. Which combinations...
- 14M.1.hl.TZ2.12: Which molecule is trigonal bipyramidal in shape? A. ...
- 14M.2.hl.TZ2.4e: The two oxygen-oxygen bonds in ozone are in fact of equal length. Deduce why this is the case...
- 14M.2.hl.TZ2.4d: In terms of \(\sigma \) and \(\pi \) bonds, describe the two oxygen-oxygen bonds in the Lewis...
- 14M.3.hl.TZ1.16d: Explain, with the aid of Lewis (electron-dot) structures, the difference between oxygen and...
- 14M.3.hl.TZ1.16e: One CFC, Freon-13 (chlorotrifl uoromethane), which can be used as a refrigerant, has been...
- 14M.3.sl.TZ1.17a: Describe, using chemical equations, the two-step mechanism of photochemical decomposition of...
- 14N.1.hl.TZ0.12: What is the correct number of sigma \({\text{(}}\sigma {\text{)}}\) and pi...
- 14N.3.hl.TZ0.22a: Explain how the bonding in \({{\text{O}}_{\text{2}}}\) and \({{\text{O}}_{\text{3}}}\)...
- 14N.3.hl.TZ0.22b: The chemical balance of the stratosphere is disrupted by the presence of chlorofluorocarbons...
- 14N.3.sl.TZ0.20a: Describe, using equations, the formation and depletion of ozone in the stratosphere by...
- 13N.1.hl.TZ0.13: How many sigma \((\sigma )\) and pi \((\pi )\) bonds are there in...
- 13N.2.hl.TZ0.3b: Bonding in the nitrate ion involves electron delocalization. Explain the meaning of electron...
- 13N.2.hl.TZ0.6f.ii: Xenon, although a noble gas, forms an oxide, \({\text{Xe}}{{\text{O}}_{\text{2}}}\), that has...
- 13N.3.hl.TZ0.21b: The ozone layer has also been depleted by certain pollutants that have been released into the...
- 13M.1.hl.TZ1.11: Which combination best describes the type of bonding present and the melting point of silicon...
- 13M.2.hl.TZ1.6e: Explain the delocalization of \(\pi \) electrons using the \({{\text{O}}_{\text{3}}}\)...
- 13M.2.hl.TZ1.6c.ii: Deduce the Lewis structures for \({\text{PC}}{{\text{l}}_{\text{3}}}\) and...
- 13M.2.hl.TZ1.6c.iii: Predict the shapes and the bond angles in the two molecules.
- 13M.3.hl.TZ1.E1c: Ozone and oxygen are in equilibrium in the stratosphere. Both gases absorb ultraviolet...
- 13M.3.sl.TZ1.E1a: Identify a gas that is both a greenhouse gas and a cause of ozone depletion.
- 13M.1.hl.TZ2.12: How many sigma (\(\sigma \)) and pi (\(\pi \)) bonds are there in the following molecule?
- 13M.1.hl.TZ2.13: Which species have delocalized \(\pi \) electrons? I. ...
- 13M.2.hl.TZ2.5c.iii: Identify all the different bond angles in PCl5.
- 13M.2.hl.TZ2.5c.i: Deduce the Lewis (electron dot) structure of PCl5.
- 13M.2.hl.TZ2.5c.ii: Predict the shape of this molecule, using the valence shell electron pair repulsion theory...
- 13M.3.sl.TZ2.E2b.i: Although the use of harmful CFCs is being phased out, suggest why these compounds are...
- 13M.3.sl.TZ2.E2b.ii: Discuss one advantage and two disadvantages of using hydrocarbons as alternatives to...
- 12N.2.hl.TZ0.1e: When iodine reacts with excess chlorine, \({\text{IC}}{{\text{l}}_{\text{3}}}\) can form....
- 10N.1.hl.TZ0.13: Which species have delocalized electrons? A. I and II only B. I and III only C. ...
- 10N.2.hl.TZ0.2a: (i) Explain the formation of the \(\pi \) bond. (ii) For each of the carbon atoms,...
- 09N.2.hl.TZ0.2: SF2, SF4 and SF6 have different shapes. Draw their Lewis structures and use the VSEPR theory...
- 10M.3.sl.TZ1.E3a: Stratospheric ozone is in dynamic equilibrium with oxygen. Give the equations that describe...
- 10M.3.sl.TZ2.E3: (a) Ozone decomposition can proceed photochemically. Describe, using chemical equations,...
- 09M.1.hl.TZ1.13: Which structure has delocalized \(\pi \) electrons? A. \({{\text{O}}_{\text{3}}}\) B. ...
- 09M.2.hl.TZ1.6a.ii: \({\text{NH}}_2^ - \)
- 09M.2.hl.TZ1.6a.iii: \({\text{Xe}}{{\text{F}}_{\text{4}}}\)
- 09M.2.hl.TZ1.6c.i: Compare the formation of a sigma \((\sigma )\) and a pi \((\pi )\) bond between two carbon...
- 09M.2.hl.TZ1.6a.i: \({\text{PC}}{{\text{l}}_{\text{3}}}\)
- 09M.2.hl.TZ1.6c.ii: Identify how many sigma and pi bonds are present in propene,...
- 09M.3.hl.TZ1.E4a: Explain how the dissociation of \({{\text{O}}_{\text{2}}}\) and \({{\text{O}}_{\text{3}}}\)...
- 09M.3.hl.TZ1.E4b: Use equations to describe the mechanism of ozone depletion catalysed by the...
- 09M.1.hl.TZ2.12: How many bonding pairs and lone pairs of electrons surround the sulfur atom in the...
- 09M.1.hl.TZ2.14: Which of the following best describes the formation of \(\pi \) bonds? A. They are...
- 09M.1.hl.TZ2.15: What is the hybridization of the carbon atom, and the number of \(\sigma \) and \(\pi \)...
- 09M.2.hl.TZ2.5b.i: \({\text{SiF}}_6^{2 - }\)
- 09M.2.hl.TZ2.5f.iii: Describe how \(\sigma \) and \(\pi \) bonds form.
- 09M.2.hl.TZ2.5f.iv: State the type of hybridization of the carbon and nitrogen atoms in...
- 09M.3.hl.TZ2.E2a: State the equations that represent the depletion of ozone in the stratosphere which is...
- 11M.1.hl.TZ1.11: Which species does not contain delocalized electrons? A. ...
- 11M.1.hl.TZ1.14: The Lewis structure of \({\text{S}}{{\text{O}}_{\text{2}}}\) is given below. What is the...
- 11M.2.hl.TZ1.6d: Describe the delocalization of pi (\(\pi \)) electrons and explain how this can account for...
- 11M.1.hl.TZ1.10: How many \(\sigma \) and \(\pi \) bonds are present in a molecule of propyne,...
- 11M.3.hl.TZ1.E4a: The following reactions take place in the ozone layer by the absorption of ultraviolet...
- 11M.3.hl.TZ1.E4b: CFCs and \({\text{N}}{{\text{O}}_{\text{x}}}\) are pollutants responsible for the depletion...
- 11M.1.hl.TZ2.14: Which species does not have delocalized electrons? A. \({\text{NO}}_3^ - \) B. ...
- 11M.1.hl.TZ2.13: How many sigma and pi bonds are there in propyne,...
- 12M.1.hl.TZ2.13: Retinol (vitamin A) contains a total of 5 double bonds and 46 single bonds. Which...
- 12M.2.hl.TZ2.7a.vii: Predict and explain the bond lengths and bond strengths of the carbon-oxygen bonds in...
- 11N.2.hl.TZ0.6d.i: Compare the formation of sigma (\(\sigma \)) and pi (\(\pi \)) bonds between the carbon atoms...
- 11N.3.hl.TZ0.E5: One of the winners of the 1995 Nobel Prize in Chemistry was Paul J. Crutzen, who showed that...
- 11N.2.sl.TZ0.6c: Nitrogen monoxide, NO, is involved in the decomposition of ozone according to the following...

