| Date | May 2009 | Marks available | 3 | Reference code | 09M.2.hl.TZ2.5 |
| Level | HL | Paper | 2 | Time zone | TZ2 |
| Command term | Draw, Predict, and State | Question number | 5 | Adapted from | N/A |
Question
Nitrogen and silicon belong to different groups in the periodic table.
Draw the Lewis structures, state the shapes and predict the bond angles for the following species.
Consider the molecule \({\text{HCON}}{{\text{H}}_{\text{2}}}\).
Distinguish in terms of electronic structure, between the terms group and period.
State the maximum number of orbitals in the \(n = 2\) energy level.
\({\text{SiF}}_6^{2 - }\)
\({\text{NO}}_2^ + \)
Explain, using diagrams, why \({\text{N}}{{\text{O}}_{\text{2}}}\) is a polar molecule but \({\text{C}}{{\text{O}}_{\text{2}}}\) is a non-polar molecule.
Explain the term hybridization.
Describe how \(\sigma \) and \(\pi \) bonds form.
State the type of hybridization of the carbon and nitrogen atoms in \({\text{HCON}}{{\text{H}}_{\text{2}}}\).
Markscheme
Group: number of valence/outer energy level electrons same;
Period: electrons are in same valence/outer energy level;
Accept number of energy levels containing electrons occupied.
Accept shell for energy level.
4;
Allow the mark if the correct individual orbitals (e.g. 2s etc.) are listed.
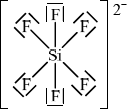 ;
;
octahedral/octahedron/square bipyramidal;
90° / 90° and 180°;
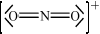 ;
;
linear;
180°;
Allow dots, crosses or lines in Lewis structures.
Penalize missing charge, missing bracket once only in (i) and (ii).
Lone pairs required for BOTH (i) and (ii).
\(N{O_2}\):
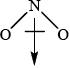
Award [1] for correct representation of the bent shape and [1] for showing the net dipole moment, or explaining it in words (unsymmetrical distribution of charge).
\(C{O_2}\):
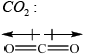
Award [1] for correct representation of the linear shape and for showing the two equal but opposite dipoles or explaining it in words (symmetrical distribution of charge).
For both species, allow either arrow or arrow with bar for representation of dipole moment.
Allow correct partial charges instead of the representation of the vector dipole moment.
Ignore incorrect bonds.
Lone pairs not needed.
mixing/joining together/combining/merging of atomic orbitals to form molecular /new orbitals / orbitals of equal energy;
\(sigma \) bond:
end-on/axial overlap with electron density between the two atoms/nuclei;
\(\pi \) bond:
sideways/parallel overlap with electron density above and below internuclear axis/\(sigma \) bond;
Marks can be scored from a suitable diagram.
Award [1 max] for stating end-on/axial overlap for \(sigma \) and sideways/parallel overlap for \(\pi \) only i.e. without mentioning electron density OR stating electron density between the two atoms/nuclei for \(sigma \) above and below internuclear axis/\(sigma \) bond for \(\pi \) i.e. without mentioning overlap.
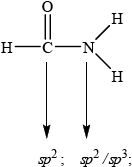
Correct answer is actually sp2 for nitrogen because of delocalization/planar geometry.
Accept sp3.
Examiners report
Part (a) was very poorly answered which was surprising at HL. Most candidates described groups correctly but only a small majority stated that for a period the electrons are in the same valence level.
Part (ii) was well answered.
For (b) VSEPR theory in general was well answered. The most common mistakes involved candidates failing to include square brackets or lone pairs of electrons or charges. Four G2 comments stated that expanded octets are not on the syllabus. However, AS 14.1.1 states explicitly that candidates should be able to predict the shape and bond angles of species of five and six negative charge centres. Four examples are included in the teachers note, including \({\text{S}}{{\text{F}}_{\text{6}}}\), but it has to emphasized again, as in previous subject reports that examples should not be confined in teaching programmes to just these four examples. Even \({\text{S}}{{\text{F}}_{\text{6}}}\) is a clear example of an expanded octet type structure, as is \({\text{SiF}}_6^{2 - }\), as asked in this question.
There were five other G2 comments again stating the fact that \({\text{NO}}_2^ + \) is off-syllabus. Based on AS 4.2.7, this example is clearly on the syllabus as the AS states that candidates should be able to predict the shape and bond angles of species of two, three and four negative charge centres. All the examples in the teachers note should be covered at a minimum in the teaching programme, but these are not the only examples.
There were seven G2 comments referring to (d); some respondents felt that the candidates had to answer the question by determining the shape of both \({\text{N}}{{\text{O}}_{\text{2}}}\) and\({\text{C}}{{\text{O}}_{\text{2}}}\) using VSEPR Theory. This is a classic example of candidates reading the question carefully and not making unnecessary assumptions in relation to what is being asked. Only three marks are allocated to this question and hence this should be another clue as to suggest that the answer can be given in a concise manner. All candidates had to do was determine the fact that both species are XY2 species (not XYZ even) and hence can only be one of two geometries, either linear or bent. \({\text{C}}{{\text{O}}_{\text{2}}}\) must be non-polar since it is a linear geometry and hence the two dipole moments cancel each other out, yielding a net dipole moment of zero. In the case of \({\text{N}}{{\text{O}}_{\text{2}}}\), the geometry must be bent, and therefore there is a net dipole moment meaning it is a polar molecule. A simple diagram of the two species with the two bond dipole moments in each case and the resultant net dipole moment (in the case of \({\text{N}}{{\text{O}}_{\text{2}}}\)) would have scored both marks. There was no need to show lone pairs of electrons or isolated electrons etc. to answer this question, as candidates were not asked to write Lewis structures etc. Some candidates wasted time here trying to work these out and even some candidates thought that there might even be a mistake in the question and tried to answer the question with \({\text{NO}}_2^ - \), because this is an example given in the teachers note in AS 14.3.1, based on delocalization.
The very best candidates did draw dipole moments, as the question did ask for diagrams, when explaining polarity, as opposed to simply a description in words.
Hybridization was usually well answered in part (ii), but sometimes candidates did not score the mark due to lack of specific subject vocabulary.
Although candidates often had some understanding of sigma and pi bonding, very few mentioned electron density in (iii).
For (iv) one G2 comment stated that the hybridization of N in \({\text{HCON}}{{\text{H}}_{\text{2}}}\) will in fact be \({\text{s}}{{\text{p}}^{\text{2}}}\) due to the planar nature of the \({\text{N}}{{\text{H}}_{\text{2}}}\) group here in this example, which is in fact correct, although it is unlikely that candidates at this level would know this. Nearly all candidates gave \({\text{s}}{{\text{p}}^{\text{3}}}\) hybridization for N, which they based on a perceived pyramidal type geometry, like in ammonia. For this reason, during GA, we decided to allow both hybridizations, even though the correct answer is actually \({\text{s}}{{\text{p}}^{\text{2}}}\) in this example.

