| Date | May 2015 | Marks available | 1 | Reference code | 15M.1.hl.TZ2.12 |
| Level | HL | Paper | 1 | Time zone | TZ2 |
| Command term | Question number | 12 | Adapted from | N/A |
Question
Which combination of shape and bond angle is correct for a molecule of xenon tetrafluoride, \({\text{Xe}}{{\text{F}}_{\text{4}}}\)?
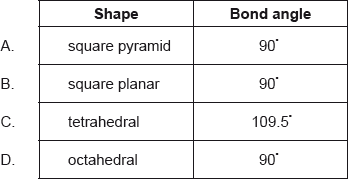
Markscheme
B
Examiners report
Whilst 70% gave the correct answer, a significant number (17%) missed the axial lone pairs and thought the molecule to be tetrahedral.
Syllabus sections
Additional higher level (AHL) » Topic 14: Chemical bonding and structure » 14.1 Covalent bonding and electron domain and molecular geometries
Show 96 related questions
- 17N.2.hl.TZ0.4a: Draw the Lewis (electron dot) structures of PF3 and PF5 and use the VSEPR theory to deduce...
- 17N.1.hl.TZ0.14: How many sigma (σ) and pi (π) bonds are present in this molecule?
- 17M.2.hl.TZ2.7c.ii: Outline, in terms of the bonding present, why the reaction conditions of halogenation are...
- 17M.2.hl.TZ2.4b.ii: Deduce one resonance structure of ozone and the corresponding formal charges on each oxygen...
- 17M.2.hl.TZ2.4b.i: Discuss the bonding in the resonance structures of ozone.
- 17M.2.hl.TZ2.3b: Deduce the Lewis (electron dot) structure and molecular geometry and the bond angles of PCl3.
- 17M.1.hl.TZ2.13: Which statement is correct? A. Sigma bonds are formed only by the combination of s...
- 17M.1.hl.TZ2.10: Which does not show resonance? A. PO43– B. C6H6 C. C6H12 D. O3
- 17M.2.hl.TZ1.8b: Suggest why the loss of ozone is an international environmental concern.
- 17M.2.hl.TZ1.8a: Formulate two equations to show how nitrogen(II) oxide, NO, catalyses the destruction of ozone.
- 17M.1.hl.TZ1.13: Which species have resonance structures? I. Ozone, O3II. Carbon dioxide, CO2III. ...
- 17M.1.hl.TZ1.12: Which combination describes the bonding and structure in benzoic acid, C6H5COOH?
- 17M.1.hl.TZ1.11: Which combination describes the PH4+ ion?
- 16N.3.hl.TZ0.22c: (i) Uranium hexafluoride, UF6, is used in the uranium enrichment process that produces fuel...
- 16N.2.hl.TZ0.5b: (i) Draw diagrams to show how sigma (σ) and pi (π) bonds are formed between atoms. (ii)...
- 16N.1.hl.TZ0.15: What is the hybridization of the numbered atoms in ethanoic acid?
- 16N.1.hl.TZ0.14: Which species has bond angles of 90°? A. AlCl4- B. \({\text{I}}\)Cl4- C. NH4+ D. SiCl4
- 16M.2.hl.TZ0.3c: One of the intermediates in the reaction between nitrogen monoxide and hydrogen is dinitrogen...
- 16M.1.hl.TZ0.14: In which group do both compounds contain delocalized...
- 16M.1.hl.TZ0.11: Which species breaks the octet rule? A. PCl3B. BF4−C. SCl4D....
- 15M.1.hl.TZ1.12: What is correct for \({\text{PC}}{{\text{l}}_{\text{5}}}\)?
- 15M.2.hl.TZ1.1e: Predict and explain the difference in carbon-oxygen bond lengths in ethanedioic acid and its...
- 15M.2.hl.TZ1.7f: Describe the formation of \(\sigma \) and \(\pi \) bonds in an alkene.
- 15M.2.hl.TZ2.9c.i: Deduce the Lewis (electron dot) structure of both molecules.
- 15M.2.hl.TZ2.9c.ii: Predict the shapes of the two molecules, giving the Br–P–Br bond angle in...
- 15M.2.hl.TZ2.9c.iii: Explain why both \({\text{PB}}{{\text{r}}_{\text{3}}}\) and...
- 15M.2.hl.TZ2.9d.iii: Describe sigma \((\sigma )\) and pi \((\pi )\) bonds between atoms. \(\sigma \)...
- 15M.2.hl.TZ2.9d.iv: Identify the number of sigma \((\sigma )\) and pi \((\pi )\) bonds present in a molecule of...
- 15M.3.hl.TZ1.20a: Explain, on a molecular level, why ozone dissociates with radiation of a longer wavelength...
- 15M.3.hl.TZ1.20b: Nitrogen(II) oxide, NO, is a primary pollutant that depletes the ozone layer. State...
- 15M.3.hl.TZ2.23b.ii: Identify and state the source of one other ozone-depleting pollutant.
- 15M.3.hl.TZ2.23b.i: Describe, by means of equations, how nitrogen(II) oxide, NO, catalyses the depletion of ozone.
- 14M.1.hl.TZ1.13: Which species contain delocalized electrons? A. I and II only B. I and III...
- 14M.1.hl.TZ1.12: The diagrams below show \(s\) and \(p\) orbitals in different positions. Which combinations...
- 14M.1.hl.TZ2.12: Which molecule is trigonal bipyramidal in shape? A. ...
- 14M.2.hl.TZ2.4e: The two oxygen-oxygen bonds in ozone are in fact of equal length. Deduce why this is the case...
- 14M.2.hl.TZ2.4d: In terms of \(\sigma \) and \(\pi \) bonds, describe the two oxygen-oxygen bonds in the Lewis...
- 14M.3.hl.TZ1.16d: Explain, with the aid of Lewis (electron-dot) structures, the difference between oxygen and...
- 14M.3.hl.TZ1.16e: One CFC, Freon-13 (chlorotrifl uoromethane), which can be used as a refrigerant, has been...
- 14M.3.sl.TZ1.17a: Describe, using chemical equations, the two-step mechanism of photochemical decomposition of...
- 14N.1.hl.TZ0.12: What is the correct number of sigma \({\text{(}}\sigma {\text{)}}\) and pi...
- 14N.3.hl.TZ0.22a: Explain how the bonding in \({{\text{O}}_{\text{2}}}\) and \({{\text{O}}_{\text{3}}}\)...
- 14N.3.hl.TZ0.22b: The chemical balance of the stratosphere is disrupted by the presence of chlorofluorocarbons...
- 14N.3.sl.TZ0.20a: Describe, using equations, the formation and depletion of ozone in the stratosphere by...
- 13N.1.hl.TZ0.13: How many sigma \((\sigma )\) and pi \((\pi )\) bonds are there in...
- 13N.2.hl.TZ0.3b: Bonding in the nitrate ion involves electron delocalization. Explain the meaning of electron...
- 13N.2.hl.TZ0.6f.ii: Xenon, although a noble gas, forms an oxide, \({\text{Xe}}{{\text{O}}_{\text{2}}}\), that has...
- 13N.3.hl.TZ0.21b: The ozone layer has also been depleted by certain pollutants that have been released into the...
- 13M.1.hl.TZ1.11: Which combination best describes the type of bonding present and the melting point of silicon...
- 13M.2.hl.TZ1.6e: Explain the delocalization of \(\pi \) electrons using the \({{\text{O}}_{\text{3}}}\)...
- 13M.2.hl.TZ1.6c.ii: Deduce the Lewis structures for \({\text{PC}}{{\text{l}}_{\text{3}}}\) and...
- 13M.2.hl.TZ1.6c.iii: Predict the shapes and the bond angles in the two molecules.
- 13M.3.hl.TZ1.E1c: Ozone and oxygen are in equilibrium in the stratosphere. Both gases absorb ultraviolet...
- 13M.3.sl.TZ1.E1a: Identify a gas that is both a greenhouse gas and a cause of ozone depletion.
- 13M.1.hl.TZ2.12: How many sigma (\(\sigma \)) and pi (\(\pi \)) bonds are there in the following molecule?
- 13M.1.hl.TZ2.13: Which species have delocalized \(\pi \) electrons? I. ...
- 13M.2.hl.TZ2.5c.iii: Identify all the different bond angles in PCl5.
- 13M.2.hl.TZ2.5c.i: Deduce the Lewis (electron dot) structure of PCl5.
- 13M.2.hl.TZ2.5c.ii: Predict the shape of this molecule, using the valence shell electron pair repulsion theory...
- 13M.3.sl.TZ2.E2b.i: Although the use of harmful CFCs is being phased out, suggest why these compounds are...
- 13M.3.sl.TZ2.E2b.ii: Discuss one advantage and two disadvantages of using hydrocarbons as alternatives to...
- 12N.2.hl.TZ0.1e: When iodine reacts with excess chlorine, \({\text{IC}}{{\text{l}}_{\text{3}}}\) can form....
- 10N.1.hl.TZ0.13: Which species have delocalized electrons? A. I and II only B. I and III only C. ...
- 10N.2.hl.TZ0.2a: (i) Explain the formation of the \(\pi \) bond. (ii) For each of the carbon atoms,...
- 09N.2.hl.TZ0.2: SF2, SF4 and SF6 have different shapes. Draw their Lewis structures and use the VSEPR theory...
- 10M.3.sl.TZ1.E3a: Stratospheric ozone is in dynamic equilibrium with oxygen. Give the equations that describe...
- 10M.3.sl.TZ2.E3: (a) Ozone decomposition can proceed photochemically. Describe, using chemical equations,...
- 09M.1.hl.TZ1.13: Which structure has delocalized \(\pi \) electrons? A. \({{\text{O}}_{\text{3}}}\) B. ...
- 09M.2.hl.TZ1.6a.ii: \({\text{NH}}_2^ - \)
- 09M.2.hl.TZ1.6a.iii: \({\text{Xe}}{{\text{F}}_{\text{4}}}\)
- 09M.2.hl.TZ1.6c.i: Compare the formation of a sigma \((\sigma )\) and a pi \((\pi )\) bond between two carbon...
- 09M.2.hl.TZ1.6a.i: \({\text{PC}}{{\text{l}}_{\text{3}}}\)
- 09M.2.hl.TZ1.6c.ii: Identify how many sigma and pi bonds are present in propene,...
- 09M.3.hl.TZ1.E4a: Explain how the dissociation of \({{\text{O}}_{\text{2}}}\) and \({{\text{O}}_{\text{3}}}\)...
- 09M.3.hl.TZ1.E4b: Use equations to describe the mechanism of ozone depletion catalysed by the...
- 09M.1.hl.TZ2.12: How many bonding pairs and lone pairs of electrons surround the sulfur atom in the...
- 09M.1.hl.TZ2.14: Which of the following best describes the formation of \(\pi \) bonds? A. They are...
- 09M.1.hl.TZ2.15: What is the hybridization of the carbon atom, and the number of \(\sigma \) and \(\pi \)...
- 09M.2.hl.TZ2.5b.i: \({\text{SiF}}_6^{2 - }\)
- 09M.2.hl.TZ2.5f.iii: Describe how \(\sigma \) and \(\pi \) bonds form.
- 09M.2.hl.TZ2.5f.iv: State the type of hybridization of the carbon and nitrogen atoms in...
- 09M.3.hl.TZ2.E2a: State the equations that represent the depletion of ozone in the stratosphere which is...
- 11M.1.hl.TZ1.11: Which species does not contain delocalized electrons? A. ...
- 11M.1.hl.TZ1.14: The Lewis structure of \({\text{S}}{{\text{O}}_{\text{2}}}\) is given below. What is the...
- 11M.2.hl.TZ1.6d: Describe the delocalization of pi (\(\pi \)) electrons and explain how this can account for...
- 11M.1.hl.TZ1.10: How many \(\sigma \) and \(\pi \) bonds are present in a molecule of propyne,...
- 11M.3.hl.TZ1.E4a: The following reactions take place in the ozone layer by the absorption of ultraviolet...
- 11M.3.hl.TZ1.E4b: CFCs and \({\text{N}}{{\text{O}}_{\text{x}}}\) are pollutants responsible for the depletion...
- 11M.1.hl.TZ2.14: Which species does not have delocalized electrons? A. \({\text{NO}}_3^ - \) B. ...
- 11M.1.hl.TZ2.13: How many sigma and pi bonds are there in propyne,...
- 12M.1.hl.TZ2.13: Retinol (vitamin A) contains a total of 5 double bonds and 46 single bonds. Which...
- 12M.2.hl.TZ2.4: Draw the Lewis structures, predict the shape and deduce the bond angles for xenon...
- 12M.2.hl.TZ2.7a.vii: Predict and explain the bond lengths and bond strengths of the carbon-oxygen bonds in...
- 11N.2.hl.TZ0.6d.i: Compare the formation of sigma (\(\sigma \)) and pi (\(\pi \)) bonds between the carbon atoms...
- 11N.3.hl.TZ0.E5: One of the winners of the 1995 Nobel Prize in Chemistry was Paul J. Crutzen, who showed that...
- 11N.2.sl.TZ0.6c: Nitrogen monoxide, NO, is involved in the decomposition of ozone according to the following...

