| Date | May 2014 | Marks available | 2 | Reference code | 14M.3.hl.TZ1.16 |
| Level | HL | Paper | 3 | Time zone | TZ1 |
| Command term | Explain | Question number | 16 | Adapted from | N/A |
Question
Ozone prevents UV radiation emitted from the Sun reaching the surface of the Earth.
Explain, with the aid of Lewis (electron-dot) structures, the difference between oxygen and ozone in terms of the energy required to dissociate both molecules.
Oxygen: Ozone:
One CFC, Freon-13 (chlorotrifl uoromethane), which can be used as a refrigerant, has been phased out by the Montreal Protocol. Describe, using equations, the mechanism of the catalysis of ozone depletion by this particular CFC.
Markscheme
Oxygen: correctly drawn Lewis structure and Ozone: correctly drawn Lewis structure;
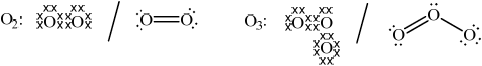
Allow any combination of dots, x’s or lines to represent electron pairs.
Allow representation of two resonance structures for ozone.
oxygen has a higher bond order than ozone and oxygen needs more energy to dissociate / OWTTE;
Exact bond orders of 2 for oxygen and 1.5/1 and 2 for ozone may be given for first statement in M2.
Do not award M2 if incorrect bond orders are stated for either species.
\({\text{C}}{{\text{F}}_{\text{3}}}{\text{Cl (}} + {\text{UV/}}hf{\text{/}}hv{\text{)}} \to {\text{C}}{{\text{F}}_{\text{3}}} \bullet + {\text{Cl}} \bullet \);
\({\text{Cl}} \bullet + {{\text{O}}_3} \to {\text{ClO}} \bullet + {{\text{O}}_2}\);
\({\text{ClO}} \bullet + {{\text{O}}_3} \to {\text{Cl}} \bullet + {\text{2}}{{\text{O}}_2}\);
Accept \(ClO \bullet + O \bullet \to {O_2} + Cl \bullet \) for M3.
Allow representation of radicals without \( \bullet \) if consistent throughout.
Penalize inconsistency of radical representations once only in E16.
Examiners report
(a) and (b) were well answered though the weaker students in (b) simply stated that \({{\text{C}}_{\text{2}}}{{\text{F}}_{\text{6}}}\) contains no chlorine – no credit was awarded for this. (c) (i) was generally well done though temperature inversion often was not correctly described. Part (ii) was very poorly answered and most only scored one mark. PANs continue to be a real challenge for candidates and similar to previous examination papers candidate performance here was very poor. In (d) the most common mistake was the sight of two lone pairs instead of one on the central oxygen in ozone. (e) was well done however, though some inconsistency of radical symbols was common.
(a) and (b) were well answered though the weaker students in (b) simply stated that \({{\text{C}}_{\text{2}}}{{\text{F}}_{\text{6}}}\) contains no chlorine – no credit was awarded for this. (c) (i) was generally well done though temperature inversion often was not correctly described. Part (ii) was very poorly answered and most only scored one mark. PANs continue to be a real challenge for candidates and similar to previous examination papers candidate performance here was very poor. In (d) the most common mistake was the sight of two lone pairs instead of one on the central oxygen in ozone. (e) was well done however, though some inconsistency of radical symbols was common.

