| Date | May 2015 | Marks available | 4 | Reference code | 15M.2.hl.TZ2.9 |
| Level | HL | Paper | 2 | Time zone | TZ2 |
| Command term | Predict | Question number | 9 | Adapted from | N/A |
Question
Consider the structure and bonding in \({\text{MgC}}{{\text{l}}_{\text{2}}}\) and \({\text{PC}}{{\text{l}}_{\text{3}}}\).
Consider the molecules \({\text{PB}}{{\text{r}}_{\text{3}}}\) and \({\text{S}}{{\text{F}}_{\text{4}}}\).
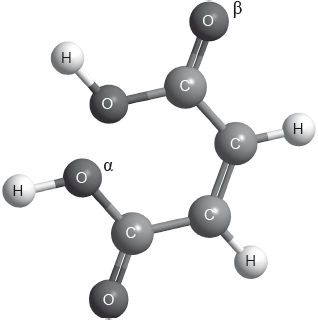
The structure of cis-but-2-ene-1,4-dioic acid is shown below.
State and explain the electrical conductivities of these two chloride compounds in their liquid state.
Suggest, giving your reasons, the approximate pH values of the solutions formed by adding each chloride compound separately to distilled water.
\({\text{MgC}}{{\text{l}}_{\text{2}}}\)
\({\text{PC}}{{\text{l}}_{\text{3}}}\)
Identify the acid-base character of the oxides of each of the elements from sodium to chlorine in period 3.
State the equations for the separate reactions of sodium oxide and phosphorus(V) oxide with water.
Deduce the Lewis (electron dot) structure of both molecules.
Predict the shapes of the two molecules, giving the Br–P–Br bond angle in \({\text{PB}}{{\text{r}}_{\text{3}}}\) and the F–S–F bond angles in \({\text{S}}{{\text{F}}_{\text{4}}}\).
Explain why both \({\text{PB}}{{\text{r}}_{\text{3}}}\) and \({\text{S}}{{\text{F}}_{\text{4}}}\) are polar.
Describe the covalent bond between carbon and hydrogen in the molecule above and how it is formed.
Deduce the hybridization of the oxygen atoms labelled \(\alpha \) and \(\beta \).
\(\alpha \):
\(\beta \):
Describe sigma \((\sigma )\) and pi \((\pi )\) bonds between atoms.
\(\sigma \) bond:
\(\pi \) bond:
Identify the number of sigma \((\sigma )\) and pi \((\pi )\) bonds present in a molecule of cis-but-2-ene-1,4-dioic acid.
Markscheme
\({\text{MgC}}{{\text{l}}_{\text{2}}}\) conducts electricity and \({\text{PC}}{{\text{l}}_{\text{3}}}\) does not;
\({\text{MgC}}{{\text{l}}_{\text{2}}}\) is ionic and \({\text{PC}}{{\text{l}}_{\text{3}}}\) is covalent/molecular;
ions/charged particles can move in \({\text{MgC}}{{\text{l}}_{\text{2}}}\) / no free charged particles in \({\text{PC}}{{\text{l}}_{\text{3}}}\);
Award [1 max] if all three points correct for one substance but not other.
\(MgC{l_{\text{2}}}\):
\(4 \leqslant {\text{pH}} \leqslant 6.9\);
high charge density/high charge and small size of \({\text{M}}{{\text{g}}^{2 + }}\) makes \({[{\text{Mg}}{({{\text{H}}_{\text{2}}}{\text{O}})_{\text{6}}}{\text{]}}^{2 + }}\) hydrolyse / polarizes water to produce \({{\text{H}}^ + }\);
\(PC{l_3}\):
\(0 \leqslant {\text{pH}} \leqslant 4\);
(reacts with water to) form \({\text{H}}{{\text{C}}_{\text{l}}}{\text{/}}{{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{3}}}\);
Do not accept \(H3P{O_4}\).
Na, Mg (oxides): basic
Al (oxide): amphoteric
Do not accept amphiprotic.
Si to Cl (oxides): acidic
Award [2] for all three listed sets correct.
Award [1] for one or two listed sets correct.
Award [1] for stating oxides become more acidic towards right/Cl or more basic towards left/Na.
Do not penalize if reference is to Ar instead of Cl.
Do not penalize for incorrect formulas of oxides.
\({\text{N}}{{\text{a}}_2}{\text{O}}({\text{s}}) + {{\text{H}}_2}{\text{O}}({\text{l}}) \to {\text{2NaOH(aq)}}\);
\({{\text{P}}_4}{{\text{O}}_{10}}({\text{s}}) + {\text{6}}{{\text{H}}_2}{\text{O}}({\text{l}}) \to {\text{4}}{{\text{H}}_3}{\text{P}}{{\text{O}}_4}{\text{(aq)}}\);
Ignore state symbols.
Accept \({P_2}{O_5}(s) + 3{H_2}O(l) \to 2{H_3}P{O_4}(aq)\).
Do not award marks if incorrect formulas of the oxides are used.

Penalize lone pairs missing on Br and F once only.
Accept any combination of lines, dots or crosses to represent electron pairs.
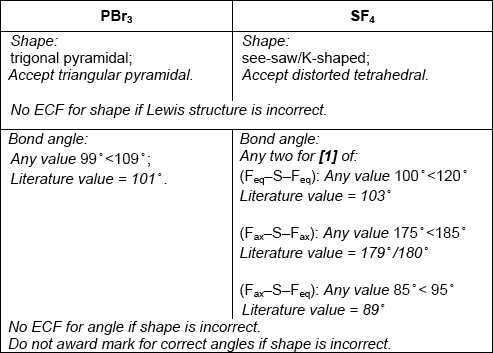
P–Br and S–F bonds are polar / bonds in both molecules are polar;
non-symmetrical distribution of electron cloud / polar bonds/dipoles do not cancel because of non-symmetrical shape;
M2 may also be scored with a suitable diagram showing the vectorial addition of the individual S–F dipole moments to show a net dipole moment centred along the axis between the \({F_{eq}}\)–S–\({F_{eq}}\) bond.
EITHER
(electrostatic) attraction between (positively charged) nuclei and a pair of electrons;
formed as a result of electron sharing (between the carbon and hydrogen nuclei);
OR
sigma bond formed by overlap of atomic orbitals;
s orbital from H and \({\text{p/s}}{{\text{p}}^{\text{2}}}\) from carbon;
\(\alpha \): \({\text{s}}{{\text{p}}^{\text{3}}}\) and \(\beta \): \({\text{s}}{{\text{p}}^{\text{2}}}\);
Accept if numbers are given as subscripts.
\(\sigma \) bond:
end-on / axial overlap of two orbitals;
\(\pi \) bond:
sideways overlap of two (parallel) p orbitals;
Accept suitable diagrams for both marks.
11 \(\sigma \) and 3 \(\pi \);
Examiners report
Most candidates knew about the relative conductivities of magnesium chloride and phosphorous trichloride and were able to relate it to bonding. The third mark was however more problematic as many continue to equate conductivity to mobile electrons rather than ions. The pH of solutions of aqueous chlorides was not generally well known with only a small number of candidates gaining full marks. An explanation of the acidity of magnesium in terms of the charge density of the \({\text{M}}{{\text{g}}^{2 + }}\) ion proved to be particularly challenging. One teacher commented that the reaction of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and water is not mentioned in the guide but it is included in the teacher note to Assessment statement 13.1.1 of the current guide (although it is not in the new guide which will be assessed for the first time in May 2016). The acidity of the period 3 oxides was generally well known but many struggled to give balanced equations to describe the reactions of sodium and phosphorous(V) oxide with water, with many confusing the reaction of sodium oxide with that of sodium giving hydrogen as a product. Most candidates were able to give correct Lewis structure and shapes and bond angles but marks were lost, as in previous session due to missing lone pairs either on the central atoms or the Br and F atoms. It should be noted that it is difficult to award ECF marks in these questions so students need to avoid careless errors. Many struggled to give a complete explanation of the polarity of the two compounds as although the molecule was identified as being asymmetrical, few stated that the P–Br and S–F bonds are polar. Only a minority of students stated that a covalent bond was an attraction between nuclei and a pair of electrons and many were unable to identify the s-orbital from hydrogen and the \({\text{p/s}}{{\text{p}}^{\text{2}}}\) orbital from carbon as the overlapping orbitals in the covalent bond. The hybridisation of oxygen was generally well known as was sigma and pi bonding.
Most candidates knew about the relative conductivities of magnesium chloride and phosphorous trichloride and were able to relate it to bonding. The third mark was however more problematic as many continue to equate conductivity to mobile electrons rather than ions. The pH of solutions of aqueous chlorides was not generally well known with only a small number of candidates gaining full marks. An explanation of the acidity of magnesium in terms of the charge density of the \({\text{M}}{{\text{g}}^{2 + }}\) ion proved to be particularly challenging. One teacher commented that the reaction of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and water is not mentioned in the guide but it is included in the teacher note to Assessment statement 13.1.1 of the current guide (although it is not in the new guide which will be assessed for the first time in May 2016). The acidity of the period 3 oxides was generally well known but many struggled to give balanced equations to describe the reactions of sodium and phosphorous(V) oxide with water, with many confusing the reaction of sodium oxide with that of sodium giving hydrogen as a product. Most candidates were able to give correct Lewis structure and shapes and bond angles but marks were lost, as in previous session due to missing lone pairs either on the central atoms or the Br and F atoms. It should be noted that it is difficult to award ECF marks in these questions so students need to avoid careless errors. Many struggled to give a complete explanation of the polarity of the two compounds as although the molecule was identified as being asymmetrical, few stated that the P–Br and S–F bonds are polar. Only a minority of students stated that a covalent bond was an attraction between nuclei and a pair of electrons and many were unable to identify the s-orbital from hydrogen and the \({\text{p/s}}{{\text{p}}^{\text{2}}}\) orbital from carbon as the overlapping orbitals in the covalent bond. The hybridisation of oxygen was generally well known as was sigma and pi bonding.
Most candidates knew about the relative conductivities of magnesium chloride and phosphorous trichloride and were able to relate it to bonding. The third mark was however more problematic as many continue to equate conductivity to mobile electrons rather than ions. The pH of solutions of aqueous chlorides was not generally well known with only a small number of candidates gaining full marks. An explanation of the acidity of magnesium in terms of the charge density of the \({\text{M}}{{\text{g}}^{2 + }}\) ion proved to be particularly challenging. One teacher commented that the reaction of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and water is not mentioned in the guide but it is included in the teacher note to Assessment statement 13.1.1 of the current guide (although it is not in the new guide which will be assessed for the first time in May 2016). The acidity of the period 3 oxides was generally well known but many struggled to give balanced equations to describe the reactions of sodium and phosphorous(V) oxide with water, with many confusing the reaction of sodium oxide with that of sodium giving hydrogen as a product. Most candidates were able to give correct Lewis structure and shapes and bond angles but marks were lost, as in previous session due to missing lone pairs either on the central atoms or the Br and F atoms. It should be noted that it is difficult to award ECF marks in these questions so students need to avoid careless errors. Many struggled to give a complete explanation of the polarity of the two compounds as although the molecule was identified as being asymmetrical, few stated that the P–Br and S–F bonds are polar. Only a minority of students stated that a covalent bond was an attraction between nuclei and a pair of electrons and many were unable to identify the s-orbital from hydrogen and the \({\text{p/s}}{{\text{p}}^{\text{2}}}\) orbital from carbon as the overlapping orbitals in the covalent bond. The hybridisation of oxygen was generally well known as was sigma and pi bonding.
Most candidates knew about the relative conductivities of magnesium chloride and phosphorous trichloride and were able to relate it to bonding. The third mark was however more problematic as many continue to equate conductivity to mobile electrons rather than ions. The pH of solutions of aqueous chlorides was not generally well known with only a small number of candidates gaining full marks. An explanation of the acidity of magnesium in terms of the charge density of the \({\text{M}}{{\text{g}}^{2 + }}\) ion proved to be particularly challenging. One teacher commented that the reaction of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and water is not mentioned in the guide but it is included in the teacher note to Assessment statement 13.1.1 of the current guide (although it is not in the new guide which will be assessed for the first time in May 2016). The acidity of the period 3 oxides was generally well known but many struggled to give balanced equations to describe the reactions of sodium and phosphorous(V) oxide with water, with many confusing the reaction of sodium oxide with that of sodium giving hydrogen as a product. Most candidates were able to give correct Lewis structure and shapes and bond angles but marks were lost, as in previous session due to missing lone pairs either on the central atoms or the Br and F atoms. It should be noted that it is difficult to award ECF marks in these questions so students need to avoid careless errors. Many struggled to give a complete explanation of the polarity of the two compounds as although the molecule was identified as being asymmetrical, few stated that the P–Br and S–F bonds are polar. Only a minority of students stated that a covalent bond was an attraction between nuclei and a pair of electrons and many were unable to identify the s-orbital from hydrogen and the \({\text{p/s}}{{\text{p}}^{\text{2}}}\) orbital from carbon as the overlapping orbitals in the covalent bond. The hybridisation of oxygen was generally well known as was sigma and pi bonding.
Most candidates knew about the relative conductivities of magnesium chloride and phosphorous trichloride and were able to relate it to bonding. The third mark was however more problematic as many continue to equate conductivity to mobile electrons rather than ions. The pH of solutions of aqueous chlorides was not generally well known with only a small number of candidates gaining full marks. An explanation of the acidity of magnesium in terms of the charge density of the \({\text{M}}{{\text{g}}^{2 + }}\) ion proved to be particularly challenging. One teacher commented that the reaction of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and water is not mentioned in the guide but it is included in the teacher note to Assessment statement 13.1.1 of the current guide (although it is not in the new guide which will be assessed for the first time in May 2016). The acidity of the period 3 oxides was generally well known but many struggled to give balanced equations to describe the reactions of sodium and phosphorous(V) oxide with water, with many confusing the reaction of sodium oxide with that of sodium giving hydrogen as a product. Most candidates were able to give correct Lewis structure and shapes and bond angles but marks were lost, as in previous session due to missing lone pairs either on the central atoms or the Br and F atoms. It should be noted that it is difficult to award ECF marks in these questions so students need to avoid careless errors. Many struggled to give a complete explanation of the polarity of the two compounds as although the molecule was identified as being asymmetrical, few stated that the P–Br and S–F bonds are polar. Only a minority of students stated that a covalent bond was an attraction between nuclei and a pair of electrons and many were unable to identify the s-orbital from hydrogen and the \({\text{p/s}}{{\text{p}}^{\text{2}}}\) orbital from carbon as the overlapping orbitals in the covalent bond. The hybridisation of oxygen was generally well known as was sigma and pi bonding.
Most candidates knew about the relative conductivities of magnesium chloride and phosphorous trichloride and were able to relate it to bonding. The third mark was however more problematic as many continue to equate conductivity to mobile electrons rather than ions. The pH of solutions of aqueous chlorides was not generally well known with only a small number of candidates gaining full marks. An explanation of the acidity of magnesium in terms of the charge density of the \({\text{M}}{{\text{g}}^{2 + }}\) ion proved to be particularly challenging. One teacher commented that the reaction of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and water is not mentioned in the guide but it is included in the teacher note to Assessment statement 13.1.1 of the current guide (although it is not in the new guide which will be assessed for the first time in May 2016). The acidity of the period 3 oxides was generally well known but many struggled to give balanced equations to describe the reactions of sodium and phosphorous(V) oxide with water, with many confusing the reaction of sodium oxide with that of sodium giving hydrogen as a product. Most candidates were able to give correct Lewis structure and shapes and bond angles but marks were lost, as in previous session due to missing lone pairs either on the central atoms or the Br and F atoms. It should be noted that it is difficult to award ECF marks in these questions so students need to avoid careless errors. Many struggled to give a complete explanation of the polarity of the two compounds as although the molecule was identified as being asymmetrical, few stated that the P–Br and S–F bonds are polar. Only a minority of students stated that a covalent bond was an attraction between nuclei and a pair of electrons and many were unable to identify the s-orbital from hydrogen and the \({\text{p/s}}{{\text{p}}^{\text{2}}}\) orbital from carbon as the overlapping orbitals in the covalent bond. The hybridisation of oxygen was generally well known as was sigma and pi bonding.
Most candidates knew about the relative conductivities of magnesium chloride and phosphorous trichloride and were able to relate it to bonding. The third mark was however more problematic as many continue to equate conductivity to mobile electrons rather than ions. The pH of solutions of aqueous chlorides was not generally well known with only a small number of candidates gaining full marks. An explanation of the acidity of magnesium in terms of the charge density of the \({\text{M}}{{\text{g}}^{2 + }}\) ion proved to be particularly challenging. One teacher commented that the reaction of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and water is not mentioned in the guide but it is included in the teacher note to Assessment statement 13.1.1 of the current guide (although it is not in the new guide which will be assessed for the first time in May 2016). The acidity of the period 3 oxides was generally well known but many struggled to give balanced equations to describe the reactions of sodium and phosphorous(V) oxide with water, with many confusing the reaction of sodium oxide with that of sodium giving hydrogen as a product. Most candidates were able to give correct Lewis structure and shapes and bond angles but marks were lost, as in previous session due to missing lone pairs either on the central atoms or the Br and F atoms. It should be noted that it is difficult to award ECF marks in these questions so students need to avoid careless errors. Many struggled to give a complete explanation of the polarity of the two compounds as although the molecule was identified as being asymmetrical, few stated that the P–Br and S–F bonds are polar. Only a minority of students stated that a covalent bond was an attraction between nuclei and a pair of electrons and many were unable to identify the s-orbital from hydrogen and the \({\text{p/s}}{{\text{p}}^{\text{2}}}\) orbital from carbon as the overlapping orbitals in the covalent bond. The hybridisation of oxygen was generally well known as was sigma and pi bonding.
Most candidates knew about the relative conductivities of magnesium chloride and phosphorous trichloride and were able to relate it to bonding. The third mark was however more problematic as many continue to equate conductivity to mobile electrons rather than ions. The pH of solutions of aqueous chlorides was not generally well known with only a small number of candidates gaining full marks. An explanation of the acidity of magnesium in terms of the charge density of the \({\text{M}}{{\text{g}}^{2 + }}\) ion proved to be particularly challenging. One teacher commented that the reaction of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and water is not mentioned in the guide but it is included in the teacher note to Assessment statement 13.1.1 of the current guide (although it is not in the new guide which will be assessed for the first time in May 2016). The acidity of the period 3 oxides was generally well known but many struggled to give balanced equations to describe the reactions of sodium and phosphorous(V) oxide with water, with many confusing the reaction of sodium oxide with that of sodium giving hydrogen as a product. Most candidates were able to give correct Lewis structure and shapes and bond angles but marks were lost, as in previous session due to missing lone pairs either on the central atoms or the Br and F atoms. It should be noted that it is difficult to award ECF marks in these questions so students need to avoid careless errors. Many struggled to give a complete explanation of the polarity of the two compounds as although the molecule was identified as being asymmetrical, few stated that the P–Br and S–F bonds are polar. Only a minority of students stated that a covalent bond was an attraction between nuclei and a pair of electrons and many were unable to identify the s-orbital from hydrogen and the \({\text{p/s}}{{\text{p}}^{\text{2}}}\) orbital from carbon as the overlapping orbitals in the covalent bond. The hybridisation of oxygen was generally well known as was sigma and pi bonding.
Most candidates knew about the relative conductivities of magnesium chloride and phosphorous trichloride and were able to relate it to bonding. The third mark was however more problematic as many continue to equate conductivity to mobile electrons rather than ions. The pH of solutions of aqueous chlorides was not generally well known with only a small number of candidates gaining full marks. An explanation of the acidity of magnesium in terms of the charge density of the \({\text{M}}{{\text{g}}^{2 + }}\) ion proved to be particularly challenging. One teacher commented that the reaction of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and water is not mentioned in the guide but it is included in the teacher note to Assessment statement 13.1.1 of the current guide (although it is not in the new guide which will be assessed for the first time in May 2016). The acidity of the period 3 oxides was generally well known but many struggled to give balanced equations to describe the reactions of sodium and phosphorous(V) oxide with water, with many confusing the reaction of sodium oxide with that of sodium giving hydrogen as a product. Most candidates were able to give correct Lewis structure and shapes and bond angles but marks were lost, as in previous session due to missing lone pairs either on the central atoms or the Br and F atoms. It should be noted that it is difficult to award ECF marks in these questions so students need to avoid careless errors. Many struggled to give a complete explanation of the polarity of the two compounds as although the molecule was identified as being asymmetrical, few stated that the P–Br and S–F bonds are polar. Only a minority of students stated that a covalent bond was an attraction between nuclei and a pair of electrons and many were unable to identify the s-orbital from hydrogen and the \({\text{p/s}}{{\text{p}}^{\text{2}}}\) orbital from carbon as the overlapping orbitals in the covalent bond. The hybridisation of oxygen was generally well known as was sigma and pi bonding.
Most candidates knew about the relative conductivities of magnesium chloride and phosphorous trichloride and were able to relate it to bonding. The third mark was however more problematic as many continue to equate conductivity to mobile electrons rather than ions. The pH of solutions of aqueous chlorides was not generally well known with only a small number of candidates gaining full marks. An explanation of the acidity of magnesium in terms of the charge density of the \({\text{M}}{{\text{g}}^{2 + }}\) ion proved to be particularly challenging. One teacher commented that the reaction of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and water is not mentioned in the guide but it is included in the teacher note to Assessment statement 13.1.1 of the current guide (although it is not in the new guide which will be assessed for the first time in May 2016). The acidity of the period 3 oxides was generally well known but many struggled to give balanced equations to describe the reactions of sodium and phosphorous(V) oxide with water, with many confusing the reaction of sodium oxide with that of sodium giving hydrogen as a product. Most candidates were able to give correct Lewis structure and shapes and bond angles but marks were lost, as in previous session due to missing lone pairs either on the central atoms or the Br and F atoms. It should be noted that it is difficult to award ECF marks in these questions so students need to avoid careless errors. Many struggled to give a complete explanation of the polarity of the two compounds as although the molecule was identified as being asymmetrical, few stated that the P–Br and S–F bonds are polar. Only a minority of students stated that a covalent bond was an attraction between nuclei and a pair of electrons and many were unable to identify the s-orbital from hydrogen and the \({\text{p/s}}{{\text{p}}^{\text{2}}}\) orbital from carbon as the overlapping orbitals in the covalent bond. The hybridisation of oxygen was generally well known as was sigma and pi bonding.
Most candidates knew about the relative conductivities of magnesium chloride and phosphorous trichloride and were able to relate it to bonding. The third mark was however more problematic as many continue to equate conductivity to mobile electrons rather than ions. The pH of solutions of aqueous chlorides was not generally well known with only a small number of candidates gaining full marks. An explanation of the acidity of magnesium in terms of the charge density of the \({\text{M}}{{\text{g}}^{2 + }}\) ion proved to be particularly challenging. One teacher commented that the reaction of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and water is not mentioned in the guide but it is included in the teacher note to Assessment statement 13.1.1 of the current guide (although it is not in the new guide which will be assessed for the first time in May 2016). The acidity of the period 3 oxides was generally well known but many struggled to give balanced equations to describe the reactions of sodium and phosphorous(V) oxide with water, with many confusing the reaction of sodium oxide with that of sodium giving hydrogen as a product. Most candidates were able to give correct Lewis structure and shapes and bond angles but marks were lost, as in previous session due to missing lone pairs either on the central atoms or the Br and F atoms. It should be noted that it is difficult to award ECF marks in these questions so students need to avoid careless errors. Many struggled to give a complete explanation of the polarity of the two compounds as although the molecule was identified as being asymmetrical, few stated that the P–Br and S–F bonds are polar. Only a minority of students stated that a covalent bond was an attraction between nuclei and a pair of electrons and many were unable to identify the s-orbital from hydrogen and the \({\text{p/s}}{{\text{p}}^{\text{2}}}\) orbital from carbon as the overlapping orbitals in the covalent bond. The hybridisation of oxygen was generally well known as was sigma and pi bonding.

