| Date | May 2009 | Marks available | 3 | Reference code | 09M.2.hl.TZ1.6 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | Draw | Question number | 6 | Adapted from | N/A |
Question
Draw the Lewis structures, state the shape and predict the bond angles for the following species.
Consider the following Born-Haber cycle:
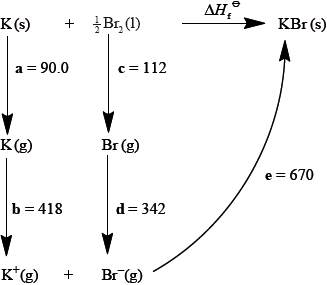
The magnitudes for each of the enthalpy changes (a to e) are given in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) but their signs (+ or –) have been omitted.
\({\text{PC}}{{\text{l}}_{\text{3}}}\)
\({\text{NH}}_2^ - \)
\({\text{Xe}}{{\text{F}}_{\text{4}}}\)
State the names for the enthalpy changes c and d.
Deduce which two of the enthalpy changes a to e have negative signs.
Determine the value for the enthalpy of formation of potassium bromide.
Explain why the quantitative value for the lattice enthalpy of calcium bromide is larger than the value for the lattice enthalpy of potassium bromide.
Compare the formation of a sigma \((\sigma )\) and a pi \((\pi )\) bond between two carbon atoms in a molecule.
Identify how many sigma and pi bonds are present in propene, \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}\).
Deduce all the bond angles present in propene.
Explain how the concept of hybridization can be used to explain the bonding in the triple bond present in propyne.
Markscheme
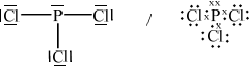 ;
;
trigonal pyramid;
in the range of 100–108°;
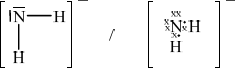 ;
;
Must include minus sign for the mark.
bent/V–shaped;
in the range of 100–106°;
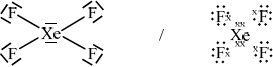 ;
;
square planar;
90°;
Penalize once only if electron pairs are missed off outer atoms.
c: atomization (enthalpy);
d: electron affinity;
d and e;
\(\Delta {H_{\text{f}}} = 90.0 + 418 + 112 + (-342) + ( - 670)\);
\( = - 392{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\);
\({\text{C}}{{\text{a}}^{2 + }}\) is smaller than \({{\text{K}}^ + }\) and \({\text{C}}{{\text{a}}^{2 + }}\) has more charge than \({{\text{K}}^ + }\) / \({\text{C}}{{\text{a}}^{2 + }}\) has a greater charge density;
so the attractive forces between the ions are stronger;
Do not accept ‘stronger ionic bonds’
Award [1 max] if reference is made to atoms or molecules instead of ions.
sigma bonds are formed by end on/axial overlap of orbitals with electron density between the two atoms/nuclei;
pi bonds are formed by sideways overlap of parallel p orbitals with electron density above and below internuclear axis/\(\sigma \) bond;
Accept suitably annotated diagrams
8 sigma/\(\sigma \) ;
1 pi/\(\pi \) ;
109°/109.5°;
120°;
sp hybridization;
1 sigma and 2 pi;
sigma bond formed by overlap between the two sp hybrid orbitals (on each of the two carbon atoms) / pi bonds formed by overlap between remaining p orbitals (on each of the two carbon atoms) / diagram showing 2 sp hybrid orbitals and 2 p orbitals;
Examiners report
This question was the most popular of the Section B questions. Part (a) was generally well answered with many candidates drawing clear Lewis structures and applying their knowledge of VSEPR theory well. Common errors included the omission of lone electron pairs on outer atoms, and the omission of a bracket and charge on the ion. Incorrect angular values were common. Some candidates described shapes and bond angles in terms of the ‘parent shape’. Good candidates explained the answers well and scored full marks. Weaker candidates simply wrote two answers; for example, ‘tetrahedral bent’ and could not be awarded marks.
This question was the most popular of the Section B questions. Part (a) was generally well answered with many candidates drawing clear Lewis structures and applying their knowledge of VSEPR theory well. Common errors included the omission of lone electron pairs on outer atoms, and the omission of a bracket and charge on the ion. Incorrect angular values were common. Some candidates described shapes and bond angles in terms of the ‘parent shape’. Good candidates explained the answers well and scored full marks. Weaker candidates simply wrote two answers; for example, ‘tetrahedral bent’ and could not be awarded marks.
This question was the most popular of the Section B questions. Part (a) was generally well answered with many candidates drawing clear Lewis structures and applying their knowledge of VSEPR theory well. Common errors included the omission of lone electron pairs on outer atoms, and the omission of a bracket and charge on the ion. Incorrect angular values were common. Some candidates described shapes and bond angles in terms of the ‘parent shape’. Good candidates explained the answers well and scored full marks. Weaker candidates simply wrote two answers; for example, ‘tetrahedral bent’ and could not be awarded marks.
In part (b) many candidates incorrectly identified the process converting liquid bromine molecules to gaseous bromine atoms as vaporization.
Deducing the enthalpy changes with negative signs proved challenging for many although, with follow through marks credit was earned for the calculation of the enthalpy of formation of potassium bromide.
Some teachers commented on the G2 forms that the energy cycle diagram was strange, however, the stages of the Born-Haber cycle were clearly given and candidates should be familiar with those.
Very few candidates could explain why calcium bromide has a larger lattice enthalpy than potassium bromide. Many referred to atoms instead of ions, and tried to answer this in terms of the electronegativity of the metals.
Part (c) was answered well by some candidates who produced clear and well annotated diagrams as part of their answers. Many candidates however omitted mention of orbitals when trying to describe the formation of sigma and pi bonds or to explain hybridization. There were many diagrams which had no annotations and were difficult to interpret.
Part (c) was answered well by some candidates who produced clear and well annotated diagrams as part of their answers. Many candidates however omitted mention of orbitals when trying to describe the formation of sigma and pi bonds or to explain hybridization. There were many diagrams which had no annotations and were difficult to interpret.
Part (c) was answered well by some candidates who produced clear and well annotated diagrams as part of their answers. Many candidates however omitted mention of orbitals when trying to describe the formation of sigma and pi bonds or to explain hybridization. There were many diagrams which had no annotations and were difficult to interpret.
Part (c) was answered well by some candidates who produced clear and well annotated diagrams as part of their answers. Many candidates however omitted mention of orbitals when trying to describe the formation of sigma and pi bonds or to explain hybridization. There were many diagrams which had no annotations and were difficult to interpret.

