| Date | November 2009 | Marks available | 1 | Reference code | 09N.3.sl.TZ0.A2 |
| Level | SL | Paper | 3 | Time zone | TZ0 |
| Command term | Identify | Question number | A2 | Adapted from | N/A |
Question
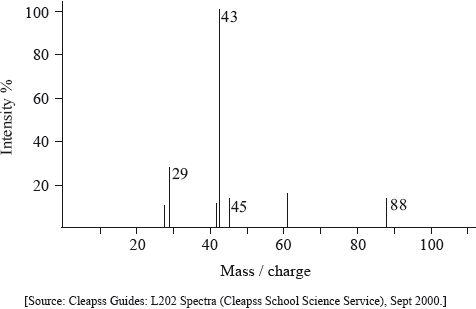
The mass spectrum of an unknown compound, X, of empirical formula C2H4O is shown below.
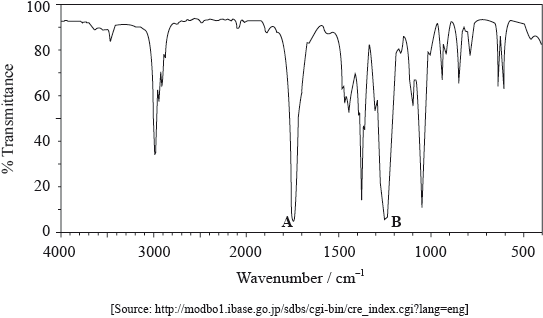
The IR spectrum of X is shown below.
Determine the relative molecular mass of X from the mass spectrum and deduce the formula of the molecular ion.
Identify a fragment which gives rise to the peak at m/z = 29.
Comment on the absence of a peak at m/z = 59.
Use Table 17 of the Data Booklet to identify the bonds which correspond to the absorptions A and B.
A:
B:
Deduce the name of the functional group present in X.
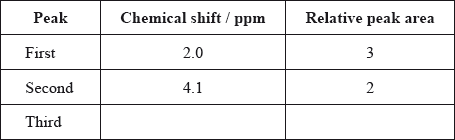
The \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of X shows three peaks. State the information that can be obtained from the number of peaks.
The \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of X includes peaks at 2.0 and 4.1 ppm. Use Table 18 of the Data Booklet to suggest the chemical shift of the third peak and state its relative peak area. Show your answers in the table below.
Deduce a possible structure for X that is consistent with the mass, IR and \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectra.
Markscheme
88;
Do not award mark if units are given.
\({{\text{C}}_4}{{\text{H}}_8}{\text{O}}_2^ + \);
\({\text{C}}{{\text{H}}_3}{\text{CH}}_2^ + /{{\text{C}}_2}{\text{H}}_5^ + /{\text{CH}}{{\text{O}}^ + }\);
Only penalize once for missing charge in (a) (i) and (ii).
C2H3O2 produced has no charge / fragment produced after loss of C2H5 from molecular ion has no charge;
Accept fragment(s) too unstable, fragment breaks up etc.
Do not accept answers with reference to 13C/14C isotopes and peak at m/z = 61.
Do not accept C2H3O2+ / C3H7O+ does not exist.
A: C=O and B: C–O;
No mark if two bonds are given for A or B.
Ignore names if incorrect.
ester;
Do not accept COO.
the number of different hydrogen/proton environments / OWTTE;
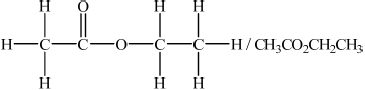 ;
;
Examiners report
In (a), although most identified the formulas of the molecular ion in (i) and the fragment in (ii), the positive charge was often missing.
In (a), although most identified the formulas of the molecular ion in (i) and the fragment in (ii), the positive charge was often missing.
Very few candidates were able to explain the absence of a peak at m/z = 29 in (iii) in the mass spectrum. This is because the fragment produced after loss of C2H5+ has no charge.
A significant number referred to the C–F bond in (b), even though the empirical formula of X given in the question contained no fluorine.
A significant number referred to the C–F bond in (b), even though the empirical formula of X given in the question contained no fluorine.
Part (c) was generally well answered, although with some errors in the table in (ii) and the structure of a carboxylic acid in (iii).
Part (c) was generally well answered, although with some errors in the table in (ii) and the structure of a carboxylic acid in (iii).
Part (c) was generally well answered, although with some errors in the table in (ii) and the structure of a carboxylic acid in (iii).

