| Date | May 2017 | Marks available | 3 | Reference code | 17M.2.hl.TZ1.6 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | Deduce | Question number | 6 | Adapted from | N/A |
Question
This question is about carbon and chlorine compounds.
Ethane, \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}\), reacts with chlorine in sunlight. State the type of this reaction and the name of the mechanism by which it occurs.
Formulate equations for the two propagation steps and one termination step in the formation of chloroethane from ethane.
Deduce the splitting patterns in the 1H NMR spectrum of C2H5Cl.
Explain why tetramethylsilane (TMS) is often used as a reference standard in 1H NMR.
One possible product, X, of the reaction of ethane with chlorine has the following composition by mass:
carbon: 24.27%, hydrogen: 4.08%, chlorine: 71.65%
Determine the empirical formula of the product.
The mass and 1H NMR spectra of product X are shown below. Deduce, giving your reasons, its structural formula and hence the name of the compound.
When the product X is reacted with NaOH in a hot alcoholic solution, C2H3Cl is formed. State the role of the reactant NaOH other than as a nucleophile.
Chloroethene, \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{3}}}{\text{Cl}}\), can undergo polymerization. Draw a section of the polymer with three repeating units.
Markscheme
substitution AND «free-»radical
OR
substitution AND chain
Award [1] for “«free-»radical substitution” or “SR” written anywhere in the answer.
[1 mark]
Two propagation steps:
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}} + \bullet {\text{Cl}} \to {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}} \bullet + {\text{HCl}}\)
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}} \bullet + {\text{C}}{{\text{l}}_{\text{2}}} \to {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{Cl}} + \bullet {\text{Cl}}\)
One termination step:
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}} \bullet + {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}} \bullet \to {{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}\)
OR
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}} \bullet + \bullet {\text{Cl}} \to {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{Cl}}\)
OR
\( \bullet {\text{Cl}} + \bullet {\text{Cl}} \to {\text{C}}{{\text{l}}_{\text{2}}}\)
Accept radical without \( \bullet \) if consistent throughout.
Allow ECF for incorrect radicals produced in propagation step for M3.
[3 marks]
triplet AND quartet
[1 mark]
chemical shift/signal outside range of common chemical shift/signal
strong signal/12/all H atoms in same environment
OR
singlet/no splitting of the signal
volatile/easily separated/easily removed
OR
inert/stabl
contains three common NMR nuclei/1H and 13C and 29Si
Do not accept chemical shift = 0.
[2 marks]
\({\text{C}} = \frac{{24.27}}{{12.01}} = 2.021\) AND \({\text{H}} = \frac{{4.08}}{{1.01}} = 4.04\) AND \({\text{Cl}} = \frac{{71.65}}{{35.45}} = 2.021\)
«hence» CH2Cl
Accept \(\frac{{24.27}}{{12.01}}\) : \(\frac{{4.08}}{{1.01}}\) : \(\frac{{71.65}}{{35.45}}.\)
Do not accept C2H4Cl2.
Award [2] for correct final answer.
[2 marks]
molecular ion peak(s) «about» m/z 100 AND «so» C2H4Cl2 «isotopes of Cl»
two signals «in 1H NMR spectrum» AND «so» CH3CHCl2
OR
«signals in» 3:1 ratio «in 1H NMR spectrum» AND «so» CH3CHCl2
OR
one doublet and one quartet «in 1H NMR spectrum» AND «so» CH3CHCl2
1,1-dichloroethane
Accept “peaks” for “signals”.
Allow ECF for a correct name for M3 if an incorrect chlorohydrocarbon is identified.
[3 marks]
base
OR
proton acceptor
[1 mark]
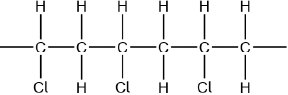
Continuation bonds must be shown.
Ignore square brackets and “n”.
Accept 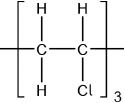 .
.
Accept other versions of the polymer, such as head to head and head to tail.
Accept condensed structure provided all C to C bonds are shown (as single).
[1 mark]

