| Date | November 2013 | Marks available | 1 | Reference code | 13N.3.sl.TZ0.2 |
| Level | SL | Paper | 3 | Time zone | TZ0 |
| Command term | Suggest | Question number | 2 | Adapted from | N/A |
Question
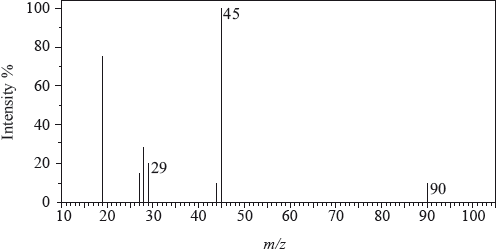
The mass spectrum of an unknown acidic compound, X, with empirical formula \({\text{C}}{{\text{H}}_{\text{2}}}{\text{O}}\), is shown below.
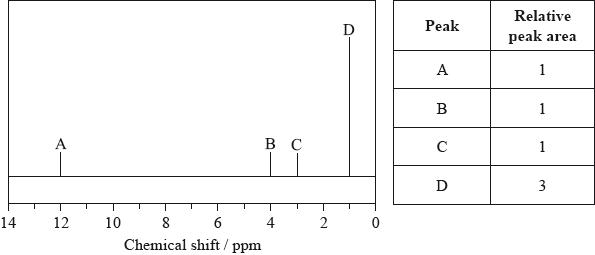
The low-resolution \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of X shows four peaks. A simplified representation is shown alongside a table with relative peak areas.
Determine the relative molecular mass, to the nearest integer, of the compound from the mass spectrum and deduce the formula of the molecular ion.
Deduce the formula of the fragment responsible for the peak at 45.
Deduce the formula of the fragment responsible for the peak at 29.
Identify the group responsible for the peak at D.
Suggest a possible structure for X.
Markscheme
90;
\({{\text{C}}_3}{{\text{H}}_6}{\text{O}}_3^ + \);
Penalize missing positive charge of ion only once in (a).
\({\text{COO}}{{\text{H}}^ + }\);
Accept C2H5O+.
Penalize missing positive charge of ion only once in (a).
\({\text{CH}}{{\text{O}}^ + }/{\text{CO}}{{\text{H}}^ + }\);
Accept C2H5+/CH3CH2+.
Penalize missing positive charge of ion only once in (a).
\({\text{C}}{{\text{H}}_3}\)/methyl;
\({\text{C}}{{\text{H}}_3}{\text{CH(OH)COOH}}\);
Allow full or condensed structural formula.
Examiners report
The Relative Molecular Mass was determined correctly but, curiously, the positive charge was omitted from the molecular ion even though it was given in parts (ii) and (iii). Part (b) was answered well although some candidates introduced a phenyl group in the structure. In part (c), the explanations were reasonable although candidates need to realize that the molecule is already vibrating; it now vibrates more. Many realized that there is a change in the dipole moment of the molecule.
As far as possible examiners use actual, rather than modified, spectra. The large peak at m/z = 19 is caused by the presence of \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\) and that at m/z = 15 is very unstable. Candidates, in general, had little difficulty interpreting the mass spectrum.
The Relative Molecular Mass was determined correctly but, curiously, the positive charge was omitted from the molecular ion even though it was given in parts (ii) and (iii). Part (b) was answered well although some candidates introduced a phenyl group in the structure. In part (c), the explanations were reasonable although candidates need to realize that the molecule is already vibrating; it now vibrates more. Many realized that there is a change in the dipole moment of the molecule.
As far as possible examiners use actual, rather than modified, spectra. The large peak at m/z = 19 is caused by the presence of \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\) and that at m/z = 15 is very unstable. Candidates, in general, had little difficulty interpreting the mass spectrum.
The Relative Molecular Mass was determined correctly but, curiously, the positive charge was omitted from the molecular ion even though it was given in parts (ii) and (iii). Part (b) was answered well although some candidates introduced a phenyl group in the structure. In part (c), the explanations were reasonable although candidates need to realize that the molecule is already vibrating; it now vibrates more. Many realized that there is a change in the dipole moment of the molecule.
As far as possible examiners use actual, rather than modified, spectra. The large peak at m/z = 19 is caused by the presence of \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\) and that at m/z = 15 is very unstable. Candidates, in general, had little difficulty interpreting the mass spectrum.
The Relative Molecular Mass was determined correctly but, curiously, the positive charge was omitted from the molecular ion even though it was given in parts (ii) and (iii). Part (b) was answered well although some candidates introduced a phenyl group in the structure. In part (c), the explanations were reasonable although candidates need to realize that the molecule is already vibrating; it now vibrates more. Many realized that there is a change in the dipole moment of the molecule.
As far as possible examiners use actual, rather than modified, spectra. The large peak at m/z = 19 is caused by the presence of \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\) and that at m/z = 15 is very unstable. Candidates, in general, had little difficulty interpreting the mass spectrum.
The Relative Molecular Mass was determined correctly but, curiously, the positive charge was omitted from the molecular ion even though it was given in parts (ii) and (iii). Part (b) was answered well although some candidates introduced a phenyl group in the structure. In part (c), the explanations were reasonable although candidates need to realize that the molecule is already vibrating; it now vibrates more. Many realized that there is a change in the dipole moment of the molecule.
As far as possible examiners use actual, rather than modified, spectra. The large peak at m/z = 19 is caused by the presence of \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\) and that at m/z = 15 is very unstable. Candidates, in general, had little difficulty interpreting the mass spectrum.

