| Date | May 2011 | Marks available | 2 | Reference code | 11M.3.sl.TZ1.A2 |
| Level | SL | Paper | 3 | Time zone | TZ1 |
| Command term | Deduce | Question number | A2 | Adapted from | N/A |
Question
The \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of X with molecular formula \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{\text{O}}\) is shown below.
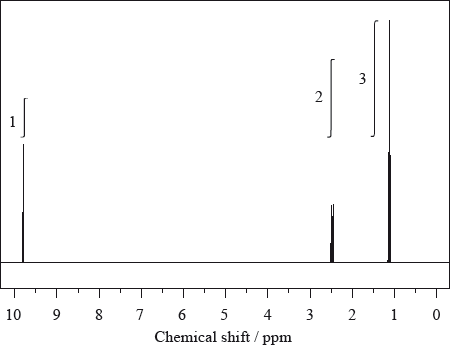
The infrared and mass spectra for X were also recorded.
Deduce which of the following compounds is X and explain your answer.
\({\text{C}}{{\text{H}}_{\text{3}}}\)–CO–\({\text{C}}{{\text{H}}_{\text{3}}}\) \({\text{C}}{{\text{H}}_{\text{3}}}\)–\({\text{C}}{{\text{H}}_{\text{2}}}\)–CHO \({\text{C}}{{\text{H}}_{\text{2}}}\)–CH–\({\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\)
Compound:
Explanation:
Deduce which one of the peaks in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of X would also occur in the spectrum of one of the other isomers, giving your reasoning.
Apart from absorptions due to C–C and C–H bonds, suggest one absorption, in wavenumbers, that would be present in the infrared spectrum.
Apart from absorptions due to C–C and C–H bonds, suggest one absorption, in wavenumbers, absent in this infrared spectrum but present in one of the other compounds shown in part (a).
Suggest the formulas and m/z values of two species that would be detected in the mass spectrum.
Species:
m/z:
Species:
m/z:
Markscheme
Compound:
\({\text{C}}{{\text{H}}_{\text{3}}}\)–\({\text{C}}{{\text{H}}_{\text{2}}}\)–CHO;
Explanation:
only this compound would give 3 peaks / OWTTE;
only this compound has H–atoms in 3 different chemical environments / OWTTE;
only this compound has protons in ratio 3:2:1 in each environment / OWTTE
only this compound would give a peak in the 9.4–10 ppm region / OWTTE;
2.5 ppm peak;
\({\text{C}}{{\text{H}}_{\text{3}}}\)–CO–\({\text{C}}{{\text{H}}_{\text{3}}}\) also has hydrogen atoms on a carbon next to the >C=O group;
1700–1750 \({\text{c}}{{\text{m}}^{ - 1}}\) (>C=O);
1610–1680 \({\text{c}}{{\text{m}}^{ - 1}}\) (>C=C<) / 3200–3600 \({\text{c}}{{\text{m}}^{ - 1}}\) (–O–H);
\({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{{\text{O}}^ + }\) and m/z = 58;
\({{\text{C}}_{\text{2}}}{\text{H}}_5^ + \) and m/z = 29;
\({\text{CH}}{{\text{O}}^ + }\) and m/z = 29;
\({\text{CH}}_3^ + \) and m/z = 15;
Penalize missing + sign once only.
Examiners report
Quite a few candidates demonstrated some skill in interpreting the nmr spectrum in terms of the groups present and could identify features that were likely to be present and absent in the in the infra-red and mass spectra, though in the latter many omitted the charges on the ions. Candidates gave too little thought to predicting features of the nmr spectra of the other compounds, concentrating on the hydrocarbon group without taking into account the neighbouring functional group.
Quite a few candidates demonstrated some skill in interpreting the nmr spectrum in terms of the groups present and could identify features that were likely to be present and absent in the in the infra-red and mass spectra, though in the latter many omitted the charges on the ions. Candidates gave too little thought to predicting features of the nmr spectra of the other compounds, concentrating on the hydrocarbon group without taking into account the neighbouring functional group.
Quite a few candidates demonstrated some skill in interpreting the nmr spectrum in terms of the groups present and could identify features that were likely to be present and absent in the in the infra-red and mass spectra, though in the latter many omitted the charges on the ions. Candidates gave too little thought to predicting features of the nmr spectra of the other compounds, concentrating on the hydrocarbon group without taking into account the neighbouring functional group.
Quite a few candidates demonstrated some skill in interpreting the nmr spectrum in terms of the groups present and could identify features that were likely to be present and absent in the in the infra-red and mass spectra, though in the latter many omitted the charges on the ions. Candidates gave too little thought to predicting features of the nmr spectra of the other compounds, concentrating on the hydrocarbon group without taking into account the neighbouring functional group.
Quite a few candidates demonstrated some skill in interpreting the nmr spectrum in terms of the groups present and could identify features that were likely to be present and absent in the in the infra-red and mass spectra, though in the latter many omitted the charges on the ions. Candidates gave too little thought to predicting features of the nmr spectra of the other compounds, concentrating on the hydrocarbon group without taking into account the neighbouring functional group.

