| Date | November 2014 | Marks available | 3 | Reference code | 14N.3.sl.TZ0.1 |
| Level | SL | Paper | 3 | Time zone | TZ0 |
| Command term | State and Suggest | Question number | 1 | Adapted from | N/A |
Question
The mass spectrum and infrared (IR) spectrum of a compound are shown below.
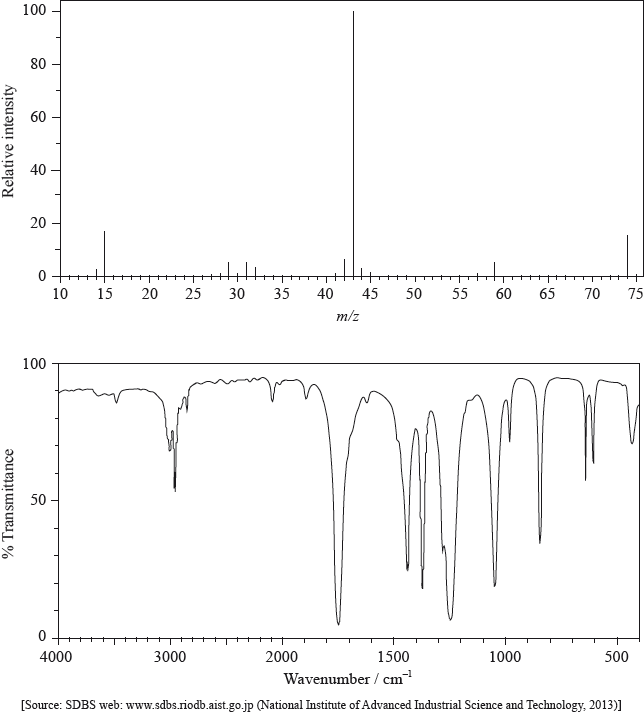
(i) State the information about this particular compound that can be derived from the mass spectrum and outline how it is found.
(ii) Suggest how the fragment with m/z = 43 is formed from the original molecule.
(i) Use the IR spectrum in the region 1600 – 1800 \({\text{c}}{{\text{m}}^{ - 1}}\) to deduce one functional group that is present in the compound and one group that is absent.
Present:
Absent:
(ii) The molecular formula of the compound is \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{{\text{O}}_{\text{2}}}\). Explain, with reference to another region of the IR spectrum, why the compound could not be propanoic acid, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH}}\).
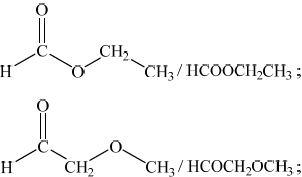
(iii) Deduce the structures of two possible isomers of propanoic acid consistent with the IR spectrum.
\(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectroscopy is often very useful in distinguishing between closely related compounds such as those above.
(i) State the region of the electromagnetic spectrum that is used in this technique.
(ii) The structures of two other closely related compounds are shown below.
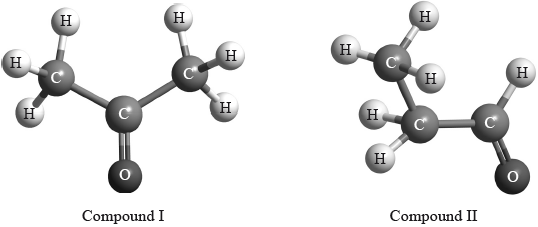
Discuss how you would expect the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectra of these two compounds to differ, using Table 18 of the Data Booklet.
Markscheme
(i) molar/molecular mass/\(M = 74{\text{ (g}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\) / relative molecular mass/\({M_r} = 74\);
peak with highest m/z (ignoring any peak attributable to \(^{{\text{13}}}{\text{C}}\)) /
found from parent/molecular ion peak;
Allow mass for m/z.
OR
compound has methyl/\({\text{C}}{{\text{H}}_{\text{3}}}\);
m/z = 15 due to \({\text{CH}}_3^ + \);
OR
compound has propyl/\({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{7}}}\)/isopropyl/\({\text{CH(C}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}\)/acetyl/\({\text{C}}{{\text{H}}_{\text{3}}}{\text{CO}}\);
m/z = 43 due to \({{\text{C}}_{\text{3}}}{\text{H}}_7^ + {\text{/CH(C}}{{\text{H}}_{\text{3}}}{\text{)}}_2^ + {\text{/C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{O}}^ + }\);
OR
compound has acetoxy/\({\text{C}}{{\text{H}}_{\text{3}}}{\text{COO}}\);
m/z = 59 due to \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CO}}{{\text{O}}^ + }\);
Fragment must contain + sign in relevant marks above.
Penalize missing charges where relevant once only in (a)(i) and (ii).
(ii) loss of \({\text{C}}{{\text{H}}_3}\)–O / loss of radical with m/z = 31 / formation of \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{3}}}{{\text{O}}^ + }{\text{/}}{{\text{C}}_{\text{3}}}{\text{H}}_7^ + {\text{/CH(C}}{{\text{H}}_{\text{3}}}{\text{)}}_2^ + \);
Penalize missing charges where relevant once only in (a)(i) and (ii).
(i) Present: carbonyl/C=O;
Do not accept aldehyde / ketone.
Accept ester/alkanoate only if m/z = 59 given in (a)(i).
Absent: carbon-carbon double bond/C=C/alkene;
(ii) no (broad) absorption at 2500-3300 \({\text{(c}}{{\text{m}}^{ - 1}}{\text{)}}\);
no O–H bond;
Award [1 max] for just stating “absorption at 1050-1410 (cm–1) / C-O bond present of alcohol/ester/ether”.
Do not accept just "C-O bond present".
Accept “peak” for “absorption”.
(iii) Any two structures from:
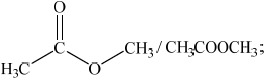
Do not penalize CH3-C connectivity here.
(i) radio (wave);
(ii) I: one signal/H environment and (compound) II: three signals/H environments;
EITHER
Award [1 max] for any two of the following chemical shift ranges from the Data Booklet:
I: 2.2-2.7 (ppm) / II: 2.2-2.7 (ppm)
II: 0.9-1.0 (ppm)
II: 9.4-10.0 (ppm);
Ranges must be associated with the correct compound (I or II).
OR
II: signal at 0.9-1.0 (ppm) and I: no such signal;
OR
II: signal at 9.4-10.0 (ppm) and I: no such signal;
Accept answers that correctly discuss differences in splitting patterns (though not on SL syllabus).
Accept “peak” for “signal”.
Do not award M2 if any contradictory chemical shift ranges are given (eg, do not allow 0.9-1.0 (ppm) for compound I).
Examiners report
In (a) (i) many candidates associated the molecular ion peak with the molar mass of the compound. Some stated incorrectly that the mass was 74, instead of stating that the molar mass was \({\text{74 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). (ii) proved problematic for a number of candidates and the difference between the loss of radicals and the positively charged fragments remaining was often lost. Greater emphasis on this difference in the teaching of this part of the curriculum on fragmentation might be worth exploring further with cohorts.
In (b) (i), most candidates identified the presence of the C=O and the absence of the C=C groups on the IR spectrum.
In (ii), although a large number of candidates stated that there is no broad absorption in the \({\text{2500 - 3300 c}}{{\text{m}}^{ - 1}}\) range, equating this to the absence of the OH bond in the IR spectrum, surprisingly some gave a more limited range of \({\text{3200 - 3600 c}}{{\text{m}}^{ - 1}}\) and associated this with the absence of hydrogen bonding. Although Table 17 of the Data Booklet does not refer specifically to the broad nature of the OH absorption in the \({\text{2500 - 3300 c}}{{\text{m}}^{ - 1}}\) range for carboxylic acids, it might be worth pointing this feature out in the teaching of IR spectroscopy, based on careful analysis of real spectral examples of carboxylic acids. In (iii), the most common mistake involved candidates drawing isomers including the OH group, which scored no marks. One G2 comment claimed that drawing an ester is not part of the syllabus. However, esters are mentioned explicitly in AS 10.1.11.
In (c) (i), nearly all identified the correct region of the EMS, namely radiowaves. (ii) proved to be a good discriminating question. Many knew that compound I had one hydrogen environment and compound II had three hydrogen environments, but only the better candidates scored the second mark. The most common mistake involved stating that compound I had a chemical shift range 0.9-1.0 ppm for the six methyl hydrogens. Many candidates clearly did not realise that the methyl groups in propanone are adjacent to the carbonyl, so hence have a higher value for the chemical shift, i.e. 2.2-2.7 ppm.

