| Date | May 2013 | Marks available | 2 | Reference code | 13M.3.sl.TZ2.A3a |
| Level | SL | Paper | 3 | Time zone | TZ2 |
| Command term | Explain | Question number | A3a | Adapted from | N/A |
Question
Three isomers of \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{{\text{O}}_{\text{2}}}\) are ethyl methanoate, methyl ethanoate and propanoic acid.
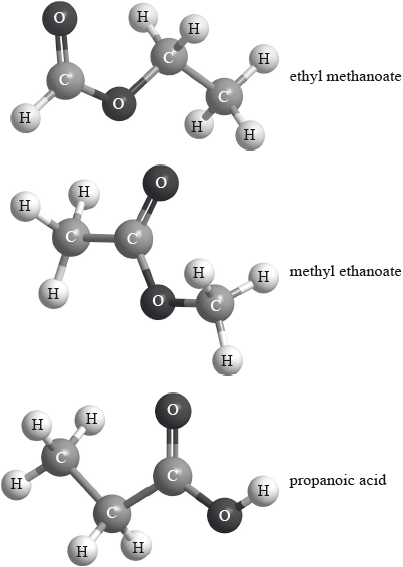
Explain which of the three compounds has a mass spectrum which contains peaks at \(m{\text{/}}z = 59\) and 44.
Markscheme
methyl ethanoate;
peak at 44 corresponds to loss of two methyl/CH3 groups (and peak at 59 the loss of the first) / the only isomer with two methyl groups / OWTTE;
Examiners report
Many students could use the spectroscopic data provided to correctly identify the compounds, with the first part of the question, dealing with mass spectrometer data, probably proving the most challenging.
Syllabus sections
Core » Topic 11: Measurement and data processing » 11.3 Spectroscopic identification of organic compounds
Show 130 related questions
- 17N.3.sl.TZ0.7b.ii: One of the two infrared (IR) spectra is that of polyethene and the other...
- 17N.2.sl.TZ0.6a.iii: Deduce the number of signals and the ratio of areas under the signals in the 1H NMR spectra...
- 17N.1.sl.TZ0.29: What information is provided by 1H NMR, MS and IR for an organic compound? I. 1H NMR:...
- 17N.3.hl.TZ0.22a.ii: Deduce which spectrum belongs to paracetamol, giving two reasons for your choice. Use section...
- 17N.3.hl.TZ0.22a.i: Both spectra show a peak at wavenumber 1700 cm–1. Identify the bond responsible for this peak.
- 17M.3.hl.TZ2.21c.i: Predict the number of different hydrogen environments in the molecule ignoring the benzene...
- 17M.3.hl.TZ2.20a.iii: Suggest two absorbances, other than the absorbances due to the ring structure and C–H bonds,...
- 17M.3.sl.TZ2.18a.ii: Deduce the wavenumber of one absorbance seen in the IR spectrum of only one of the compounds,...
- 17M.3.sl.TZ2.4: Infrared (IR) spectra can be used to distinguish between various types of plastics. Some...
- 17M.2.hl.TZ2.7a.ii: Identify the functional group that shows stretching at 1710 cm–1 in the infrared spectrum of...
- 17M.2.hl.TZ2.7a.i: Deduce what information can be obtained from the 1H NMR spectrum.
- 17M.2.sl.TZ2.8c: Identify the highest m/z value in the mass spectrum of quinone.
- 17M.2.sl.TZ2.8b: Identify the species responsible for the peak at m/z = 110 in the mass spectrum of...
- 17M.1.sl.TZ2.29: What can be deduced from the following 1H\(\,\)NMR spectrum? A. There is only one...
- 17M.1.sl.TZ2.28: Which information can be gained from an infrared (IR) spectrum? A. Ionization energy of...
- 17M.3.sl.TZ1.19d: Organic molecules can be characterized using infrared (IR) spectroscopy. Compare and...
- 17M.3.sl.TZ1.10a: Below are the IR spectra of two plastics (A and B); one is PETE, the other is low density...
- 17M.2.hl.TZ1.6c.ii: The mass and 1H NMR spectra of product X are shown below. Deduce, giving your reasons, its...
- 17M.2.sl.TZ1.5c.ii: The mass and 1H\(\,\)NMR spectra of product X are shown below. Deduce, giving your reasons,...
- 17M.1.hl.TZ1.40: Which technique is used to determine the bond lengths and bond angles of a molecule? A. ...
- 17M.1.sl.TZ1.30: What is the Index of Hydrogen Deficiency (IHD) for 1,3,5-hexatriene (C6H8)? A. 1 B. ...
- 17M.1.sl.TZ1.28: What can be determined about a molecule from the number of signals in its 1H\(\,\)NMR...
- 16N.1.sl.TZ0.29: What is always correct about the molecular ion, M+, in a mass spectrum of a compound? A. The...
- 16N.1.sl.TZ0.28: What is the index of hydrogen deficiency (IHD) for this molecule? A. 3 B. 4 C. 5 D. 6
- 16M.3.hl.TZ0.26b: Predict the number of signals and relative integration you would expect to see in the nuclear...
- 16M.3.sl.TZ0.17b: The reaction can be monitored by infrared spectroscopy. Using section 26 of the data booklet,...
- 16M.2.hl.TZ0.5b: Outline how you could use the IR spectra of compounds A and B and section 26 of the data...
- 16M.2.hl.TZ0.2c: The other monomer used in the production of polyurethane is compound Z shown below. (i)...
- 16M.2.sl.TZ0.4c: A sample of compound A was prepared in which the 12C in the CH2 group was replaced by...
- 16M.2.sl.TZ0.4b: Compound B is related to compound A. (i) State the term that is used to describe molecules...
- 16M.1.sl.TZ0.30: Which molecule has an index of hydrogen deficiency (IHD)...
- 16M.1.sl.TZ0.29: Which feature of a molecule can be determined from its 1H NMR...
- 15M.3.hl.TZ1.2a.i: The mass spectrum of A is shown below. Deduce the formula of the molecular ion from the...
- 15M.1.sl.TZ1.6: Ultraviolet radiation has a shorter wavelength than infrared radiation. How does the...
- 15M.3.sl.TZ1.2b.iii: Deduce a structural formula consistent with the data.
- 15M.2.sl.TZ2.1b.i: Determine the change in temperature, \(\Delta T\).
- 15M.3.sl.TZ1.2a.i: Determine the relative molecular mass of the compound from the mass spectrum and deduce the...
- 15M.3.sl.TZ1.2a.ii: Deduce the formulas of the fragments which give rise to peaks at \(m/z = 27\) and...
- 15M.3.sl.TZ1.2b.ii: Identify the bond responsible for the IR absorption at B.
- 15M.3.sl.TZ2.2: NMR spectroscopy is one of the most powerful analytical tools for determining molecular...
- 15M.3.sl.TZ2.3a: Deduce two features you would expect to observe in its mass spectrum.
- 15M.3.sl.TZ2.3b: Predict two features you would expect to observe in its infrared (IR) spectrum.
- 14M.3.hl.TZ2.3a: Its infrared (IR) spectrum is represented below. Deduce the bonds responsible for the...
- 14M.3.hl.TZ2.3b: The \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum recorded showed four peaks with the...
- 14M.3.hl.TZ2.3c: Deduce the fragments in the mass spectrum which correspond to the following \(m{\text{/}}z\)...
- 14M.3.hl.TZ2.3d: Deduce the structural formula of X.
- 14M.3.sl.TZ2.3a: Its infrared (IR) spectrum is represented below. Deduce the bonds responsible for the...
- 14M.3.sl.TZ2.3f: (i) Like X, 3-methylbutanoic acid is also a source of body odour. Deduce the m/z value...
- 14M.3.sl.TZ1.1b: Identify the five missing components in the following table.
- 14M.3.sl.TZ1.2a: The mass spectrum of iodoethane,...
- 14M.3.sl.TZ1.2b: Bromine contains two isotopes, \(^{{\text{79}}}{\text{Br}}\) and...
- 14M.3.sl.TZ1.4: Two students were provided with three different isomers of...
- 14M.3.sl.TZ2.3b: The \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum recorded showed four peaks with the...
- 14M.3.sl.TZ2.3c: Deduce the fragments in the mass spectrum which correspond to the following m/z...
- 14M.3.sl.TZ2.3d: Deduce the structural formula of X.
- 14M.3.sl.TZ2.3e: (i) Deduce the structural formula of Y. (ii) Predict one difference between the...
- 14N.3.hl.TZ0.1a: (i) State the information about this particular compound that can be derived from the...
- 14N.3.hl.TZ0.1b: (i) Use the IR spectrum in the region 1600 – 1800 \({\text{c}}{{\text{m}}^{ - 1}}\) to...
- 14N.3.sl.TZ0.1c: \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectroscopy is often very useful in distinguishing...
- 14N.3.sl.TZ0.1a: (i) State the information about this particular compound that can be derived from the...
- 14N.3.sl.TZ0.1b: (i) Use the IR spectrum in the region 1600 – 1800 \({\text{c}}{{\text{m}}^{ - 1}}\) to...
- 13N.3.hl.TZ0.2a.i: Determine the relative molecular mass, to the nearest integer, of the compound from the mass...
- 13N.3.hl.TZ0.2a.iii: Deduce the formula of the fragment responsible for the peak at 29.
- 13N.3.hl.TZ0.2b.i: Identify the group responsible for the peak at D.
- 13N.3.hl.TZ0.2a.ii: Deduce the formula of the fragment responsible for the peak at 45.
- 13N.3.hl.TZ0.2b.ii: Suggest a possible structure for X.
- 13N.3.sl.TZ0.2a.i: Determine the relative molecular mass, to the nearest integer, of the compound from the mass...
- 13N.3.sl.TZ0.2a.ii: Deduce the formula of the fragment responsible for the peak at 45.
- 13N.3.sl.TZ0.2a.iii: Deduce the formula of the fragment responsible for the peak at 29.
- 13N.3.sl.TZ0.2b.i: Identify the group responsible for the peak at D.
- 13N.3.sl.TZ0.2b.ii: Suggest a possible structure for X.
- 13M.3.sl.TZ1.A1c: Explain how the mass spectra of the structures in (a) can be used to distinguish between them.
- 13M.3.sl.TZ1.A1d.i: Deduce the formulas of the species with the m/z values at 86, 71 and...
- 13M.3.sl.TZ1.A1b: Explain why the infrared spectra of the structures in (a) are very similar.
- 13M.3.sl.TZ1.A1d.ii: Suggest a reason for the peak at m/z = 43 having an exceptionally high relative abundance.
- 13M.3.sl.TZ1.A3c: Identify the wavenumbers of two peaks in the infrared spectrum of compound Q, using Table 17...
- 13M.3.sl.TZ1.A3a: Identify what information from the spectrum allows the determination of the relative numbers...
- 13M.3.sl.TZ1.A3b: Deduce which of the following compounds is...
- 13M.3.sl.TZ2.A3c: Explain which of the three compounds has a \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum...
- 13M.3.sl.TZ2.A3b: Explain which of the three compounds has an infrared spectrum with a broad absorption between...
- 12N.3.hl.TZ0.A3a: Calculate the number of hydrogen atoms for peaks with chemical shifts of 2.15 and 2.4–2.5...
- 12N.3.sl.TZ0.A2b: The mass spectrum of the same compound contains strong peaks of...
- 12N.3.sl.TZ0.A2c: Using the information above, deduce the identity of the organic compound.
- 12N.3.sl.TZ0.A2d: Predict the number of peaks in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of this...
- 12N.3.sl.TZ0.A3a: State one advantage of MRI over X-ray medical imaging with reference to the electromagnetic...
- 12N.3.sl.TZ0.A3b: Outline how MRI is used to scan the human body.
- 10N.3.hl.TZ0.A2c: (i) Identify the bonds responsible for the peaks A, B and C in the IR spectrum of...
- 10N.3.sl.TZ0.A2c: (i) Identify the bonds responsible for the peaks A, B and C in the IR spectrum of...
- 09N.3.hl.TZ0.A2c.ii: Complete the table above by suggesting the chemical shift of the third peak, and state its...
- 09N.3.hl.TZ0.A2c.i: Deduce a possible structure for X that is consistent with the mass, IR and...
- 09N.3.sl.TZ0.A2a.iii: Comment on the absence of a peak at m/z = 59.
- 09N.3.sl.TZ0.A2b.ii: Deduce the name of the functional group present in X.
- 09N.3.sl.TZ0.A2c.ii: The \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of X includes peaks at 2.0 and 4.1 ppm....
- 09N.3.sl.TZ0.A2a.i: Determine the relative molecular mass of X from the mass spectrum and deduce the formula of...
- 09N.3.sl.TZ0.A2c.iii: Deduce a possible structure for X that is consistent with the mass, IR and...
- 09N.3.sl.TZ0.A1a.i: Identify the region of the electromagnetic spectrum used in...
- 09N.3.sl.TZ0.A1a.ii: Identify which of these two techniques involves higher energy radiation.
- 09N.3.sl.TZ0.A2a.ii: Identify a fragment which gives rise to the peak at m/z = 29.
- 09N.3.sl.TZ0.A2b.i: Use Table 17 of the Data Booklet to identify the bonds which correspond to the absorptions A...
- 09N.3.sl.TZ0.A2c.i: The \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of X shows three peaks. State the...
- 10M.3.sl.TZ1.A1a: (i) Identify which alcohol gives spectrum 1 and explain your answer by stating which...
- 10M.3.sl.TZ1.A1b: (i) Deduce which two of the alcohols could produce this spectrum and identify the species...
- 10M.3.sl.TZ1.A1c: Explain why the infrared spectra of all four alcohols are very similar.
- 09M.3.sl.TZ1.A1d: Consider the IR spectra of the following three...
- 09M.3.sl.TZ1.A2a: Distinguish between the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectra of 1-bromopropane and...
- 09M.3.sl.TZ2.A2b: Identify the fragments responsible for the peaks at m/z = 15 m/z = 45
- 09M.3.sl.TZ2.A2c: Identify a compound that could produce this spectrum.
- 09M.3.sl.TZ2.A3c.i: Explain why the structural formula of X cannot be:
- 09M.3.sl.TZ2.A2a: Determine the molecular formula of the compound.
- 09M.3.sl.TZ2.A3c.ii: The \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of X consists of three peaks. Deduce the...
- 11M.3.sl.TZ1.A2a: Deduce which of the following compounds is X and explain your...
- 11M.3.sl.TZ1.A2b: Deduce which one of the peaks in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of X...
- 11M.3.sl.TZ1.A2c.i: Apart from absorptions due to C–C and C–H bonds, suggest one absorption, in wavenumbers, that...
- 11M.3.sl.TZ1.A2c.ii: Apart from absorptions due to C–C and C–H bonds, suggest one absorption, in wavenumbers,...
- 11M.3.sl.TZ1.A2d: Suggest the formulas and m/z values of two species that would be detected in the mass...
- 11M.3.sl.TZ2.A2b.iii: Identify the peak at 11.5 ppm in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum.
- 11M.3.sl.TZ2.A2b.v: Deduce the structure of X.
- 11M.3.sl.TZ2.A2b.i: In the IR spectrum, identify the bond responsible for each of the absorptions labelled I, II...
- 11M.3.sl.TZ2.A2b.vi: ...
- 11M.3.sl.TZ2.A2b.ii: In the mass spectrum, deduce which fragments the m/z values at 102, 57 and 45 correspond...
- 11M.3.sl.TZ2.A2b.iv: State what information can be obtained from the integration traces in the...
- 12M.3.sl.TZ1.A1a: Identify the analytical technique that would most readily provide the additional data...
- 12M.3.sl.TZ1.A1b: (i) State what information about a molecule can be obtained from its IR spectrum. (ii) ...
- 12M.3.sl.TZ1.A1c: (i) Deduce what information can be obtained from these data. (ii) Deduce the...
- 12M.3.hl.TZ2.A3b: Another structural isomer of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\) is...
- 12M.3.sl.TZ2.A3b: Compare the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of 1-bromo-2-methylpropane with...
- 12M.3.sl.TZ2.A3a: Deduce the number of peaks in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectra of...
- 11N.3.sl.TZ0.A3a: Deduce two similarities and one difference in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\)...
- 11N.3.sl.TZ0.A3b: The mass spectrum of one of the two isomers above has significant peaks at mass to charge...
- 11N.3.sl.TZ0.F1c.i: Deduce the number of C=C bonds present in one molecule of each fatty...

