| Date | May 2014 | Marks available | 1 | Reference code | 14M.3.sl.TZ2.3 |
| Level | SL | Paper | 3 | Time zone | TZ2 |
| Command term | Deduce | Question number | 3 | Adapted from | N/A |
Question
Compound X has the molecular formula \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{{\text{O}}_{\text{3}}}\) and is found in human perspiration.
Y is an isomer of X, which contains the same functional groups.
Its infrared (IR) spectrum is represented below.
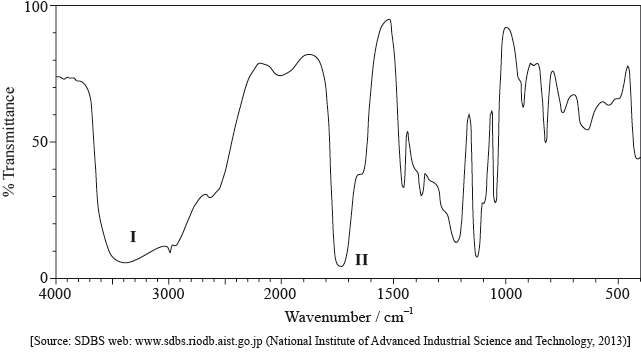
Deduce the bonds responsible for the absorptions labelled I and II.
I:
II:
The \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum recorded showed four peaks with the following chemical shift values (in ppm):
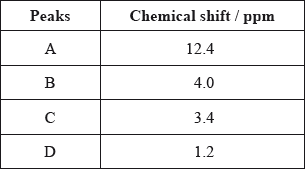
The integration trace for A:B:C:D was found to be 1:1:1:3.
Deduce what information can be obtained about the hydrogen atoms responsible for peak D at 1.2 ppm from the integration trace in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of X.
Deduce the fragments in the mass spectrum which correspond to the following m/z values.
m/z = 45:
m/z = 17:
m/z = 15:
Deduce the structural formula of X.
(i) Deduce the structural formula of Y.
(ii) Predict one difference between the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of Y and X.
(i) Like X, 3-methylbutanoic acid is also a source of body odour. Deduce the m/z value for the molecular ion peak on the mass spectrum of this compound.
(ii) Deduce the number of different chemical environments of the hydrogen atoms in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of 3-methylbutanoic acid.
Markscheme
I: O–H and II: C=O;
Do not allow CO for C=O.
Allow OH for O–H.
three hydrogens in same (chemical) environment / \({\text{C}}{{\text{H}}_{\text{3}}}\)/methyl (group);
Award [2] for all three correct, [1] for any two correct.
m/z = 45:
\({\text{COO}}{{\text{H}}^ + }/{\text{C}}{{\text{O}}_2}{{\text{H}}^ + }/{{\text{C}}_2}{{\text{H}}_5}{{\text{O}}^ + }\);
m/z = 17:
\({\text{O}}{{\text{H}}^ - }\);
m/z = 15:
\({\text{CH}}_3^ + \);
Penalize missing + once only.
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(OH)COOH}}/{\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(OH)C}}{{\text{O}}_{\text{2}}}{\text{H}}\);
Allow full or condensed structural formula.
(i) \({\text{C}}{{\text{H}}_{\text{2}}}{\text{(OH)C}}{{\text{H}}_{\text{2}}}{\text{COOH}}/{\text{HO(C}}{{\text{H}}_{\text{2}}}{{\text{)}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{2}}}{\text{H}}\);
Allow full or condensed structural formula.
(ii) different integration trace / integration trace 1:2:2:1 (in Y) / different chemical shift values / OWTTE;
(i) 102;
(ii) 4;
Examiners report
Most candidates scored this mark by identifying the bonds responsible for the absorptions.
About half the candidates were able to analyze the integration trace correctly and deduced that this was a methyl group.
Generally well answered. However, a few candidates are still forgetting to include the positive charge of the fragments of the mass spectrum.
About a third of the candidates were able to deduce the correct structural formula of X based on the evidence presented.
(i) Only few candidates deduced the correct structure for the isomer Y.
(ii) About half the candidates predicted a reasonable difference between the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectra of X and Y.
(i) More than half the candidates were able to deduce the molecular formula from the name and hence calculated the m/z value of the molecular ion peak correctly.
(ii) More than half of the candidates deduced the correct number of chemical environments in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of 3-methylbutanoic acid.

