| Date | May 2013 | Marks available | 1 | Reference code | 13M.3.sl.TZ1.A1 |
| Level | SL | Paper | 3 | Time zone | TZ1 |
| Command term | Explain | Question number | A1 | Adapted from | N/A |
Question
Compound P contains a carbonyl group (C=O) and has the molecular formula C3H6O.
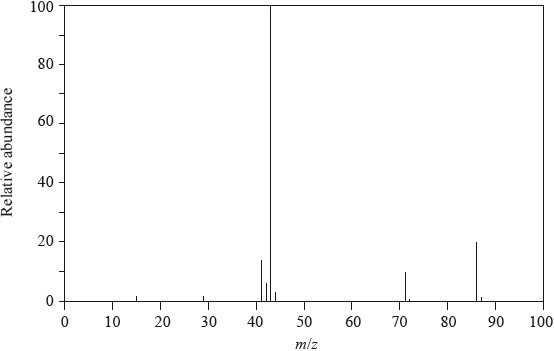
Pentan-2-one has the following mass spectrum.
Draw the two possible structures of compound P.
Explain why the infrared spectra of the structures in (a) are very similar.
Explain how the mass spectra of the structures in (a) can be used to distinguish between them.
Deduce the formulas of the species with the m/z values at 86, 71 and 43.
\(m{\text{/}}z = 86\):
\(m{\text{/}}z = 71\):
\(m{\text{/}}z = 43\):
Suggest a reason for the peak at m/z = 43 having an exceptionally high relative abundance.
Markscheme
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}}\) and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CHO}}\);
Accept full or condensed structural formulas.
Ignore incorrect names as long as structures are correct.
same/similar (types of) bonds / both contain the carbonyl group/C=O;
Do not accept same functional group.
(mass spectrum of) \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CHO}}\) contains peak at \(m{\text{/}}z = 29\) / \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}}\) does not contain peak at \(m{\text{/}}z = 29\);
(corresponding to) loss of \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\) / \({M_{\text{r}}} - {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\) / \({\text{CH}}{{\text{O}}^ + }\) / loss of CHO / \({M_{\text{r}}} - {\text{CHO}}\) / \({{\text{C}}_{\text{2}}}{\text{H}}_{\text{5}}^ + \);
OR
(mass spectrum of) \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CHO}}\) contains a (strong) peak at \(m{\text{/}}z = 57\) / \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}}\) does not contain a (strong) peak at \(m{\text{/}}z = 57\);
(corresponding to) loss of H / \({M_{\text{r}}} - {\text{H}}\) / \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}^ + }\);
Penalize missing + once only in A1.
m/z = 86: \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COCH}}_{\text{3}}^ + {\text{/}}{{\text{C}}_{\text{3}}}{{\text{H}}_{\text{7}}}{\text{COCH}}_{\text{3}}^ + {\text{/}}{{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{10}}}}{{\text{O}}^ + }\);
m/z = 71: \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}^ + }{\text{/}}{{\text{C}}_{\text{3}}}{{\text{H}}_{\text{7}}}{\text{C}}{{\text{O}}^ + }{\text{/}}{{\text{C}}_{\text{4}}}{{\text{H}}_{\text{7}}}{{\text{O}}^ + }\);
Accept CH3COCH2CH2+
m/z = 43: \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CH}}_{\text{2}}^ + {\text{ / C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{O}}^ + }{\text{ / }}{{\text{C}}_{\text{3}}}{\text{H}}_{\text{7}}^ + {\text{ / }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{3}}}{{\text{O}}^ + }\);
Penalize missing + once only in A1.
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CH}}_{\text{2}}^ + \) and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{O}}^ + }\)/two species have this mass/m/z;
Do not penalize missing + in this part.
Examiners report
The majority of candidates was able to identify the two structures in (a) and recognized that IR spectroscopy could not distinguish them easily because they contained the same types of bonds in (b).
The majority of candidates was able to identify the two structures in (a) and recognized that IR spectroscopy could not distinguish them easily because they contained the same types of bonds in (b).
Answers to part (c) were often general and did not meet the requirements. Only few candidates predicted the peaks in the mass spectrum that could be used to distinguish the two compounds.
Part (d)(i) and (ii) were answered well by about half the candidates. However, some candidates are still forgetting to include a positive charge for fragments detected in the mass spectrometer.
Part (d)(i) and (ii) were answered well by about half the candidates. However, some candidates are still forgetting to include a positive charge for fragments detected in the mass spectrometer.

