| Date | May 2014 | Marks available | 1 | Reference code | 14M.3.hl.TZ2.3 |
| Level | HL | Paper | 3 | Time zone | TZ2 |
| Command term | Deduce | Question number | 3 | Adapted from | N/A |
Question
Compound X has the molecular formula \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{{\text{O}}_{\text{3}}}\) and is found in human perspiration.
Its infrared (IR) spectrum is represented below.
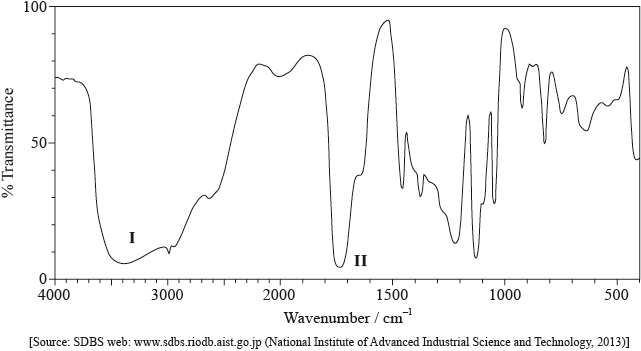
Deduce the bonds responsible for the absorptions labelled I and II.
I:
II:
The \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum recorded showed four peaks with the following chemical shift values (in ppm):
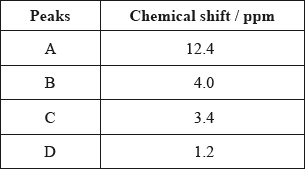
The integration trace for A:B:C:D was found to be 1:1:1:3.
Deduce what information can be obtained about the hydrogen atoms responsible for peak D at 1.2 ppm from the integration trace in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of X.
Deduce the fragments in the mass spectrum which correspond to the following \(m{\text{/}}z\) values.
\(m{\text{/}}z = 45\):
\(m{\text{/}}z = 17\):
\(m{\text{/}}z = 15\):
Deduce the structural formula of X.
Y is an isomer of X, which contains the same functional groups. Deduce the structural formula of Y.
(i) Like X, 3-methylbutanoic acid is also a source of body odour. Deduce the \(m{\text{/}}z\) value for the molecular ion peak on the mass spectrum of this compound.
(ii) Ethyl propanoate (ethyl propionate) is an isomer of 3-methylbutanoic acid. Its \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum consists of four peaks.
Deduce the ratios of the areas under each peak in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of ethyl propanoate. For each peak, deduce the range of chemical shift values (in ppm), using Table 18 of the Data Booklet, and predict the splitting pattern.
Markscheme
I: O–H and II: C=O;
Do not allow CO for C=O.
Allow OH for O–H.
three hydrogens in same (chemical) environment / CH3/methyl (group);
Award [2] for all three correct, [1] for any two correct.
\(m{\text{/}}z = 45\):
\({\text{COO}}{{\text{H}}^ + }/{\text{C}}{{\text{O}}_2}{{\text{H}}^ + }/{{\text{C}}_2}{{\text{H}}_5}{{\text{O}}^ + }\);
\(m{\text{/}}z = 17\):
\({\text{O}}{{\text{H}}^ + }\);
\(m{\text{/}}z = 15\):
\({\text{CH}}_3^ + \);
Penalize missing + once only.
\({\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(OH)COOH}}/{\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(OH)C}}{{\text{O}}_{\text{2}}}{\text{H}}\);
Allow full or condensed structural formula.
\({\text{C}}{{\text{H}}_2}{\text{(OH)C}}{{\text{H}}_2}{\text{COOH}}/{\text{HO(C}}{{\text{H}}_2}{{\text{)}}_2}{\text{COH}}\);
Allow full or condensed structural formula.
(i) 102;
(ii) 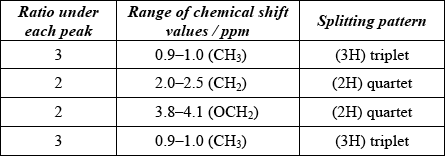
Award [3 max] for four correct rows.
Award [2 max] for any two or three correct rows and [1 max] for any correct row.
Examiners report
There were good answers to Q3 but the usual errors were encountered, such as the omission of a positive charge on mass spectrum fragments. Many were able to deduce the structure of the lactic acid although an ether was a common suggestion. It was disappointing to note that many candidates could not provide the correct \(m{\text{/}}z\) value for 3-methylbutanoic acid. Candidates found the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) information difficult although most candidates managed to give one line correctly.
There were good answers to Q3 but the usual errors were encountered, such as the omission of a positive charge on mass spectrum fragments. Many were able to deduce the structure of the lactic acid although an ether was a common suggestion. It was disappointing to note that many candidates could not provide the correct \(m{\text{/}}z\) value for 3-methylbutanoic acid. Candidates found the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) information difficult although most candidates managed to give one line correctly.
There were good answers to Q3 but the usual errors were encountered, such as the omission of a positive charge on mass spectrum fragments. Many were able to deduce the structure of the lactic acid although an ether was a common suggestion. It was disappointing to note that many candidates could not provide the correct \(m{\text{/}}z\) value for 3-methylbutanoic acid. Candidates found the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) information difficult although most candidates managed to give one line correctly.
There were good answers to Q3 but the usual errors were encountered, such as the omission of a positive charge on mass spectrum fragments. Many were able to deduce the structure of the lactic acid although an ether was a common suggestion. It was disappointing to note that many candidates could not provide the correct \(m{\text{/}}z\) value for 3-methylbutanoic acid. Candidates found the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) information difficult although most candidates managed to give one line correctly.
There were good answers to Q3 but the usual errors were encountered, such as the omission of a positive charge on mass spectrum fragments. Many were able to deduce the structure of the lactic acid although an ether was a common suggestion. It was disappointing to note that many candidates could not provide the correct \(m{\text{/}}z\) value for 3-methylbutanoic acid. Candidates found the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) information difficult although most candidates managed to give one line correctly.
There were good answers to Q3 but the usual errors were encountered, such as the omission of a positive charge on mass spectrum fragments. Many were able to deduce the structure of the lactic acid although an ether was a common suggestion. It was disappointing to note that many candidates could not provide the correct \(m{\text{/}}z\) value for 3-methylbutanoic acid. Candidates found the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) information difficult although most candidates managed to give one line correctly.

