| Date | May 2014 | Marks available | 6 | Reference code | 14M.3.sl.TZ1.4 |
| Level | SL | Paper | 3 | Time zone | TZ1 |
| Command term | Evaluate | Question number | 4 | Adapted from | N/A |
Question
Two students were provided with three different isomers of \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{{\text{O}}_{\text{2}}}\).
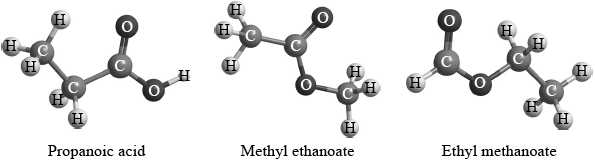
They were asked to suggest how the isomers could be distinguished and positively identified from each other using spectroscopic techniques. Student A said that they could be positively identified just from their infrared spectra. Student B said that they could be positively identified just from the number of peaks and the areas under each peak in their \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectra.
Evaluate these two claims and suggest how any possible limitations could be overcome using the same spectroscopic technique.
Student A / Infrared:
Student B / \(^{\text{1}}{\text{H}}\,{\text{NMR}}\):
Markscheme
Student A / Infrared
propanoic acid can be distinguished from the other two by the (broad) absorption at \({\text{2500–3300 c}}{{\text{m}}^{ - 1}}\) /due to –OH absorption in carboxylic acids;
not easy to distinguish between methyl ethanoate and ethyl methanoate because all absorb at \({\text{1700–1750 c}}{{\text{m}}^{ - 1}}\) / C=O / \({\text{1050–1410 c}}{{\text{m}}^{ - 1}}\) / C–O / \({\text{2850–3100 c}}{{\text{m}}^{ - 1}}\) / C–H / because they have same functional groups;
can be distinguished from the pattern in the fingerprint region / by comparing with spectra of known samples;
Student B / 1H NMR
methyl ethanoate can be distinguished from the other two as it will have two peaks of equal area (due to the two –CH3 groups);
propanoic acid and ethyl methanoate cannot be distinguished as both have three peaks / peaks in the ratio of 1:2:3;
chemical shift is also required to distinguish them;
Absorption value or name of functional group required for M1 and M2.
Examiners report
This question proved to be very challenging for most candidates as majority were unable to evaluate the two claims. Candidates appeared to have some general understanding but were often lacking depth in understanding of the two analytical techniques. The answers were often too general and most did not suggest ways to overcome limitations using the same spectroscopic techniques. Limited understanding of the directive term ‘evaluate’ along with missing the ‘same’ spectroscopic technique in the question stem, penalized the most students from scoring even part marks.

