| Date | November 2010 | Marks available | 9 | Reference code | 10N.3.hl.TZ0.A2 |
| Level | HL | Paper | 3 | Time zone | TZ0 |
| Command term | Deduce, Explain, Identify, and State | Question number | A2 | Adapted from | N/A |
Question
Infrared (IR) spectroscopy is widely used as a technique in analytical chemistry.
The IR spectrum, mass spectrum and \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of an unknown compound, Y, of molecular formula \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{{\text{O}}_{\text{2}}}\) are as follows.
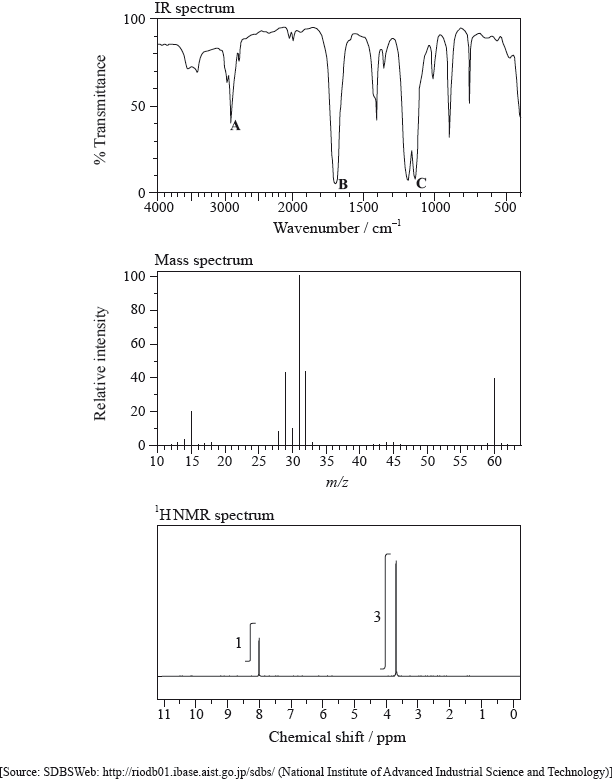
(i) Identify the bonds responsible for the peaks A, B and C in the IR spectrum of Y.
A:
B:
C:
(ii) In the mass spectrum of Y, deduce which ions the m/z values at 31 and 29 correspond to.
m/z = 31:
m/z = 29:
(iii) Identify the peaks at 3.76 and 8.07 ppm in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum.
3.76 ppm:
8.07 ppm:
(iv) State what information can be obtained from the integration trace about the hydrogen atoms responsible for the peak at 3.76 ppm in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum.
(v) Deduce the structure of Y.
(vi) Explain why tetramethylsilane (TMS) is suitable as a reference standard.
Markscheme
(i) A: C–H
B: C=O
C: C–O
Award [2] for three correct, [1] for two correct.
(ii) m/z = 31: \({\text{C}}{{\text{H}}_{\text{3}}}{{\text{O}}^ + }\);
m/z = 29: \({\text{HC}}{{\text{O}}^ + }\);
Penalize missing + charge once only.
Elements can be given in any order (i.e. OCH3+, COH+, CHO+).
(iii) 3.76 ppm: \({\text{C}}{{\text{H}}_3}{\text{O}}\) and 8.07 ppm: HCO;
Allow CH3 for CH3O.
Allow RCOOCH2 and RCOOCH3 for 3.76 ppm and RCHO for 8.07 ppm.
(iv) three hydrogens in same environment;
Allow three times as many hydrogens in this environment as for the other peak.
(v) \({\text{HC}}{{\text{O}}_2}{\text{C}}{{\text{H}}_3}{\text{ / HCOOC}}{{\text{H}}_3}\);
(vi) all (12) protons/hydrogens in same chemical environment (and hence gives 1 peak);
absorbs upfield/away from most other protons/H’s;
low boiling point/bp / volatile / easily removed from sample;
not toxic;
highly unreactive (and hence does not interfere with sample) / inert;
Examiners report
In (c) (i), most candidates were able to relate B to C=O and C to C-O, but many gave O-H for A instead of C-H. In (ii), the most common mistake was candidates omitting the + charge. In (iii), only the best candidates scored the mark. One respondent stated in a G2 form that the value of 8.07 ppm is outside the range 9.4 to 10.0 ppm given in Table 18 of the Data Booklet and that candidates are not required to know how added shielding or deshielding from neighbouring groups affects the chemical shift. This is an interesting point and it should be emphasised that the spectra used are based on real spectra and, as is pointed out clearly in Table 18, chemical shift values may vary in different solvents and conditions. This is a very important point that teachers should emphasise to candidates in the teaching programme. In this question, candidates use a combination of spectra to deduce the structure of \({\text{HCOOC}}{{\text{H}}_{\text{3}}}\). In part (ii), \({\text{HC}}{{\text{O}}^ + }\) is identified corresponding to m/z = 29. Question part (iv) was usually well done but many candidates were not able to deduce the correct structure, \({\text{HCOOC}}{{\text{H}}_{\text{3}}}\) in (v). Many candidates gave the answer as ethanoic acid. Part (vi) was usually well answered and a significant number of candidates scored both marks.

