| Date | May 2012 | Marks available | 1 | Reference code | 12M.3.sl.TZ1.A1 |
| Level | SL | Paper | 3 | Time zone | TZ1 |
| Command term | Identify | Question number | A1 | Adapted from | N/A |
Question
Analytical techniques are very useful in determining molecular structures. A compound, X, has the empirical formula \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{O}}\).
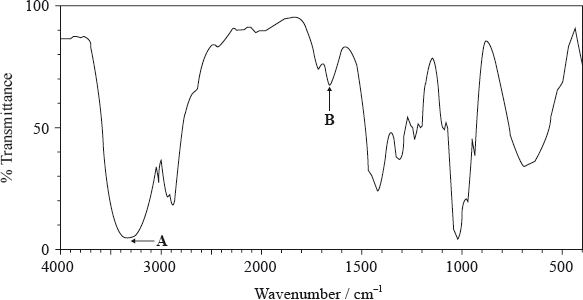
The molecular formula of X is \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{{\text{O}}_{\text{2}}}\). The information in the IR spectrum below can be used to help determine the structure of X.
The \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of X shows three peaks with relative areas of 2:1:1.
Identify the analytical technique that would most readily provide the additional data required to calculate the molecular formula of X.
(i) State what information about a molecule can be obtained from its IR spectrum.
(ii) Deduce the information obtained from absorptions A and B.
A:
B:
(iii) Comment on the absence of any major absorption in the region 1700–1750 \({\text{c}}{{\text{m}}^{ - 1}}\).
(i) Deduce what information can be obtained from these data.
(ii) Deduce the structure of X.
Markscheme
mass spectrometry/spectroscopy / MS;
(i) presence (or absence) of particular bonds;
Accept functional groups.
(ii) A: O–H/hydroxyl;
B: C=C/carbon-carbon double bond;
(iii) no C=O/carbonyl present;
(i) protons/H’s in three different chemical environments / OWTTE;
2:1:1 ratio of protons/H’s (in these environments) / OWTTE;
Accept 4:2:2
(ii) HO–\({\text{C}}{{\text{H}}_{\text{2}}}\)–CH=CH–\({\text{C}}{{\text{H}}_{\text{2}}}\)–OH / \({\text{C}}{{\text{H}}_{\text{2}}}\)=\({\text{C(C}}{{\text{H}}_{\text{2}}}{\text{OH}}{{\text{)}}_{\text{2}}}\);
Examiners report
Candidates were for the most part, able to correctly deduce the structural features from the different spectroscopic evidence but were not able to deduce the structure of X as the molecular formula was not considered.
Candidates were for the most part, able to correctly deduce the structural features from the different spectroscopic evidence but were not able to deduce the structure of X as the molecular formula was not considered.
Candidates were for the most part, able to correctly deduce the structural features from the different spectroscopic evidence but were not able to deduce the structure of X as the molecular formula was not considered.

