| Date | May 2009 | Marks available | 2 | Reference code | 09M.3.sl.TZ2.A3 |
| Level | SL | Paper | 3 | Time zone | TZ2 |
| Command term | Deduce | Question number | A3 | Adapted from | N/A |
Question
Infrared spectroscopy is an analytical technique that uses electromagnetic radiation.
The infrared spectrum of a substance, X, with empirical formula \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{\text{O}}\) is given below.
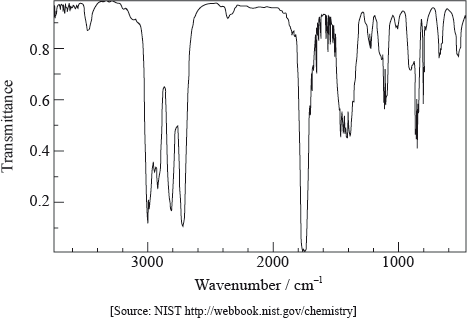
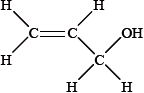
Explain why the structural formula of X cannot be:
The \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of X consists of three peaks. Deduce the structural formula of X and the relative areas under each peak.
Markscheme
absence of peak between 3200–3600 \({\text{c}}{{\text{m}}^{ - 1}}\)/above 3000 \({\text{c}}{{\text{m}}^{ - 1}}\)/peak for OH;
presence of peak between 1700–1750 \({\text{c}}{{\text{m}}^{ - 1}}\)/peak for C=O;
absence of peak between 1610–1680 \({\text{c}}{{\text{m}}^{ - 1}}\) /peak for C=C;
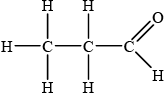 ;
;
Accept CH3CH2CHO.
3:2:1;
Ignore order
ECF if structure is incorrect only if its NMR spectrum contains three peaks.
Examiners report
Many candidates only gave at least one reason why X cannot be the given formula.
Many gave the correct formula for X, but some could not identify the relative areas under the peaks.
Syllabus sections
- 17N.3.sl.TZ0.7b.ii: One of the two infrared (IR) spectra is that of polyethene and the other...
- 17N.2.sl.TZ0.6a.iii: Deduce the number of signals and the ratio of areas under the signals in the 1H NMR spectra...
- 17N.1.sl.TZ0.29: What information is provided by 1H NMR, MS and IR for an organic compound? I. 1H NMR:...
- 17N.3.hl.TZ0.22a.ii: Deduce which spectrum belongs to paracetamol, giving two reasons for your choice. Use section...
- 17N.3.hl.TZ0.22a.i: Both spectra show a peak at wavenumber 1700 cm–1. Identify the bond responsible for this peak.
- 17M.3.hl.TZ2.21c.i: Predict the number of different hydrogen environments in the molecule ignoring the benzene...
- 17M.3.hl.TZ2.20a.iii: Suggest two absorbances, other than the absorbances due to the ring structure and C–H bonds,...
- 17M.3.sl.TZ2.18a.ii: Deduce the wavenumber of one absorbance seen in the IR spectrum of only one of the compounds,...
- 17M.3.sl.TZ2.4: Infrared (IR) spectra can be used to distinguish between various types of plastics. Some...
- 17M.2.hl.TZ2.7a.ii: Identify the functional group that shows stretching at 1710 cm–1 in the infrared spectrum of...
- 17M.2.hl.TZ2.7a.i: Deduce what information can be obtained from the 1H NMR spectrum.
- 17M.2.sl.TZ2.8c: Identify the highest m/z value in the mass spectrum of quinone.
- 17M.2.sl.TZ2.8b: Identify the species responsible for the peak at m/z = 110 in the mass spectrum of...
- 17M.1.sl.TZ2.29: What can be deduced from the following 1H\(\,\)NMR spectrum? A. There is only one...
- 17M.1.sl.TZ2.28: Which information can be gained from an infrared (IR) spectrum? A. Ionization energy of...
- 17M.3.sl.TZ1.19d: Organic molecules can be characterized using infrared (IR) spectroscopy. Compare and...
- 17M.3.sl.TZ1.10a: Below are the IR spectra of two plastics (A and B); one is PETE, the other is low density...
- 17M.2.hl.TZ1.6c.ii: The mass and 1H NMR spectra of product X are shown below. Deduce, giving your reasons, its...
- 17M.2.sl.TZ1.5c.ii: The mass and 1H\(\,\)NMR spectra of product X are shown below. Deduce, giving your reasons,...
- 17M.1.hl.TZ1.40: Which technique is used to determine the bond lengths and bond angles of a molecule? A. ...
- 17M.1.sl.TZ1.30: What is the Index of Hydrogen Deficiency (IHD) for 1,3,5-hexatriene (C6H8)? A. 1 B. ...
- 17M.1.sl.TZ1.28: What can be determined about a molecule from the number of signals in its 1H\(\,\)NMR...
- 16N.1.sl.TZ0.29: What is always correct about the molecular ion, M+, in a mass spectrum of a compound? A. The...
- 16N.1.sl.TZ0.28: What is the index of hydrogen deficiency (IHD) for this molecule? A. 3 B. 4 C. 5 D. 6
- 16M.3.hl.TZ0.26b: Predict the number of signals and relative integration you would expect to see in the nuclear...
- 16M.3.sl.TZ0.17b: The reaction can be monitored by infrared spectroscopy. Using section 26 of the data booklet,...
- 16M.2.hl.TZ0.5b: Outline how you could use the IR spectra of compounds A and B and section 26 of the data...
- 16M.2.hl.TZ0.2c: The other monomer used in the production of polyurethane is compound Z shown below. (i)...
- 16M.2.sl.TZ0.4c: A sample of compound A was prepared in which the 12C in the CH2 group was replaced by...
- 16M.2.sl.TZ0.4b: Compound B is related to compound A. (i) State the term that is used to describe molecules...
- 16M.1.sl.TZ0.30: Which molecule has an index of hydrogen deficiency (IHD)...
- 16M.1.sl.TZ0.29: Which feature of a molecule can be determined from its 1H NMR...
- 15M.3.hl.TZ1.2a.i: The mass spectrum of A is shown below. Deduce the formula of the molecular ion from the...
- 15M.1.sl.TZ1.6: Ultraviolet radiation has a shorter wavelength than infrared radiation. How does the...
- 15M.3.sl.TZ1.2b.iii: Deduce a structural formula consistent with the data.
- 15M.2.sl.TZ2.1b.i: Determine the change in temperature, \(\Delta T\).
- 15M.3.sl.TZ1.2a.i: Determine the relative molecular mass of the compound from the mass spectrum and deduce the...
- 15M.3.sl.TZ1.2a.ii: Deduce the formulas of the fragments which give rise to peaks at \(m/z = 27\) and...
- 15M.3.sl.TZ1.2b.ii: Identify the bond responsible for the IR absorption at B.
- 15M.3.sl.TZ2.2: NMR spectroscopy is one of the most powerful analytical tools for determining molecular...
- 15M.3.sl.TZ2.3a: Deduce two features you would expect to observe in its mass spectrum.
- 15M.3.sl.TZ2.3b: Predict two features you would expect to observe in its infrared (IR) spectrum.
- 14M.3.hl.TZ2.3a: Its infrared (IR) spectrum is represented below. Deduce the bonds responsible for the...
- 14M.3.hl.TZ2.3b: The \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum recorded showed four peaks with the...
- 14M.3.hl.TZ2.3c: Deduce the fragments in the mass spectrum which correspond to the following \(m{\text{/}}z\)...
- 14M.3.hl.TZ2.3d: Deduce the structural formula of X.
- 14M.3.sl.TZ2.3a: Its infrared (IR) spectrum is represented below. Deduce the bonds responsible for the...
- 14M.3.sl.TZ2.3f: (i) Like X, 3-methylbutanoic acid is also a source of body odour. Deduce the m/z value...
- 14M.3.sl.TZ1.1b: Identify the five missing components in the following table.
- 14M.3.sl.TZ1.2a: The mass spectrum of iodoethane,...
- 14M.3.sl.TZ1.2b: Bromine contains two isotopes, \(^{{\text{79}}}{\text{Br}}\) and...
- 14M.3.sl.TZ1.4: Two students were provided with three different isomers of...
- 14M.3.sl.TZ2.3b: The \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum recorded showed four peaks with the...
- 14M.3.sl.TZ2.3c: Deduce the fragments in the mass spectrum which correspond to the following m/z...
- 14M.3.sl.TZ2.3d: Deduce the structural formula of X.
- 14M.3.sl.TZ2.3e: (i) Deduce the structural formula of Y. (ii) Predict one difference between the...
- 14N.3.hl.TZ0.1a: (i) State the information about this particular compound that can be derived from the...
- 14N.3.hl.TZ0.1b: (i) Use the IR spectrum in the region 1600 – 1800 \({\text{c}}{{\text{m}}^{ - 1}}\) to...
- 14N.3.sl.TZ0.1c: \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectroscopy is often very useful in distinguishing...
- 14N.3.sl.TZ0.1a: (i) State the information about this particular compound that can be derived from the...
- 14N.3.sl.TZ0.1b: (i) Use the IR spectrum in the region 1600 – 1800 \({\text{c}}{{\text{m}}^{ - 1}}\) to...
- 13N.3.hl.TZ0.2a.i: Determine the relative molecular mass, to the nearest integer, of the compound from the mass...
- 13N.3.hl.TZ0.2a.iii: Deduce the formula of the fragment responsible for the peak at 29.
- 13N.3.hl.TZ0.2b.i: Identify the group responsible for the peak at D.
- 13N.3.hl.TZ0.2a.ii: Deduce the formula of the fragment responsible for the peak at 45.
- 13N.3.hl.TZ0.2b.ii: Suggest a possible structure for X.
- 13N.3.sl.TZ0.2a.i: Determine the relative molecular mass, to the nearest integer, of the compound from the mass...
- 13N.3.sl.TZ0.2a.ii: Deduce the formula of the fragment responsible for the peak at 45.
- 13N.3.sl.TZ0.2a.iii: Deduce the formula of the fragment responsible for the peak at 29.
- 13N.3.sl.TZ0.2b.i: Identify the group responsible for the peak at D.
- 13N.3.sl.TZ0.2b.ii: Suggest a possible structure for X.
- 13M.3.sl.TZ1.A1c: Explain how the mass spectra of the structures in (a) can be used to distinguish between them.
- 13M.3.sl.TZ1.A1d.i: Deduce the formulas of the species with the m/z values at 86, 71 and...
- 13M.3.sl.TZ1.A1b: Explain why the infrared spectra of the structures in (a) are very similar.
- 13M.3.sl.TZ1.A1d.ii: Suggest a reason for the peak at m/z = 43 having an exceptionally high relative abundance.
- 13M.3.sl.TZ1.A3c: Identify the wavenumbers of two peaks in the infrared spectrum of compound Q, using Table 17...
- 13M.3.sl.TZ1.A3a: Identify what information from the spectrum allows the determination of the relative numbers...
- 13M.3.sl.TZ1.A3b: Deduce which of the following compounds is...
- 13M.3.sl.TZ2.A3c: Explain which of the three compounds has a \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum...
- 13M.3.sl.TZ2.A3a: Explain which of the three compounds has a mass spectrum which contains peaks at...
- 13M.3.sl.TZ2.A3b: Explain which of the three compounds has an infrared spectrum with a broad absorption between...
- 12N.3.hl.TZ0.A3a: Calculate the number of hydrogen atoms for peaks with chemical shifts of 2.15 and 2.4–2.5...
- 12N.3.sl.TZ0.A2b: The mass spectrum of the same compound contains strong peaks of...
- 12N.3.sl.TZ0.A2c: Using the information above, deduce the identity of the organic compound.
- 12N.3.sl.TZ0.A2d: Predict the number of peaks in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of this...
- 12N.3.sl.TZ0.A3a: State one advantage of MRI over X-ray medical imaging with reference to the electromagnetic...
- 12N.3.sl.TZ0.A3b: Outline how MRI is used to scan the human body.
- 10N.3.hl.TZ0.A2c: (i) Identify the bonds responsible for the peaks A, B and C in the IR spectrum of...
- 10N.3.sl.TZ0.A2c: (i) Identify the bonds responsible for the peaks A, B and C in the IR spectrum of...
- 09N.3.hl.TZ0.A2c.ii: Complete the table above by suggesting the chemical shift of the third peak, and state its...
- 09N.3.hl.TZ0.A2c.i: Deduce a possible structure for X that is consistent with the mass, IR and...
- 09N.3.sl.TZ0.A2a.iii: Comment on the absence of a peak at m/z = 59.
- 09N.3.sl.TZ0.A2b.ii: Deduce the name of the functional group present in X.
- 09N.3.sl.TZ0.A2c.ii: The \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of X includes peaks at 2.0 and 4.1 ppm....
- 09N.3.sl.TZ0.A2a.i: Determine the relative molecular mass of X from the mass spectrum and deduce the formula of...
- 09N.3.sl.TZ0.A2c.iii: Deduce a possible structure for X that is consistent with the mass, IR and...
- 09N.3.sl.TZ0.A1a.i: Identify the region of the electromagnetic spectrum used in...
- 09N.3.sl.TZ0.A1a.ii: Identify which of these two techniques involves higher energy radiation.
- 09N.3.sl.TZ0.A2a.ii: Identify a fragment which gives rise to the peak at m/z = 29.
- 09N.3.sl.TZ0.A2b.i: Use Table 17 of the Data Booklet to identify the bonds which correspond to the absorptions A...
- 09N.3.sl.TZ0.A2c.i: The \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of X shows three peaks. State the...
- 10M.3.sl.TZ1.A1a: (i) Identify which alcohol gives spectrum 1 and explain your answer by stating which...
- 10M.3.sl.TZ1.A1b: (i) Deduce which two of the alcohols could produce this spectrum and identify the species...
- 10M.3.sl.TZ1.A1c: Explain why the infrared spectra of all four alcohols are very similar.
- 09M.3.sl.TZ1.A1d: Consider the IR spectra of the following three...
- 09M.3.sl.TZ1.A2a: Distinguish between the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectra of 1-bromopropane and...
- 09M.3.sl.TZ2.A2b: Identify the fragments responsible for the peaks at m/z = 15 m/z = 45
- 09M.3.sl.TZ2.A2c: Identify a compound that could produce this spectrum.
- 09M.3.sl.TZ2.A3c.i: Explain why the structural formula of X cannot be:
- 09M.3.sl.TZ2.A2a: Determine the molecular formula of the compound.
- 11M.3.sl.TZ1.A2a: Deduce which of the following compounds is X and explain your...
- 11M.3.sl.TZ1.A2b: Deduce which one of the peaks in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of X...
- 11M.3.sl.TZ1.A2c.i: Apart from absorptions due to C–C and C–H bonds, suggest one absorption, in wavenumbers, that...
- 11M.3.sl.TZ1.A2c.ii: Apart from absorptions due to C–C and C–H bonds, suggest one absorption, in wavenumbers,...
- 11M.3.sl.TZ1.A2d: Suggest the formulas and m/z values of two species that would be detected in the mass...
- 11M.3.sl.TZ2.A2b.iii: Identify the peak at 11.5 ppm in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum.
- 11M.3.sl.TZ2.A2b.v: Deduce the structure of X.
- 11M.3.sl.TZ2.A2b.i: In the IR spectrum, identify the bond responsible for each of the absorptions labelled I, II...
- 11M.3.sl.TZ2.A2b.vi: ...
- 11M.3.sl.TZ2.A2b.ii: In the mass spectrum, deduce which fragments the m/z values at 102, 57 and 45 correspond...
- 11M.3.sl.TZ2.A2b.iv: State what information can be obtained from the integration traces in the...
- 12M.3.sl.TZ1.A1a: Identify the analytical technique that would most readily provide the additional data...
- 12M.3.sl.TZ1.A1b: (i) State what information about a molecule can be obtained from its IR spectrum. (ii) ...
- 12M.3.sl.TZ1.A1c: (i) Deduce what information can be obtained from these data. (ii) Deduce the...
- 12M.3.hl.TZ2.A3b: Another structural isomer of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\) is...
- 12M.3.sl.TZ2.A3b: Compare the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of 1-bromo-2-methylpropane with...
- 12M.3.sl.TZ2.A3a: Deduce the number of peaks in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectra of...
- 11N.3.sl.TZ0.A3a: Deduce two similarities and one difference in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\)...
- 11N.3.sl.TZ0.A3b: The mass spectrum of one of the two isomers above has significant peaks at mass to charge...
- 11N.3.sl.TZ0.F1c.i: Deduce the number of C=C bonds present in one molecule of each fatty...

